J Korean Ophthalmol Soc.
2011 May;52(5):618-623. 10.3341/jkos.2011.52.5.618.
Effect of Creatine on the Survival of RGC-5 Cells under Serum Deprivation
- Affiliations
-
- 1Department of Ophthalmology, Catholic University of Daegu School of Medicine, Daegu, Korea. jwkim@cu.ac.kr
- KMID: 2214583
- DOI: http://doi.org/10.3341/jkos.2011.52.5.618
Abstract
- PURPOSE
To evaluate the protective effect of creatine on the survival of retinal ganglion cells after serum deprivation.
METHODS
RGC-5 cells were exposed to 5 mM creatine with serum-free media for 4 days. Cellular survival and mitochondrial respiratory activity were measured with MTT assay and resazurin assay, respectively. Degree of apoptosis was evaluated with vital staining using acridine orange/Hoechest 33342 and flow cytometric analysis using annexin/PI, respectively.
RESULTS
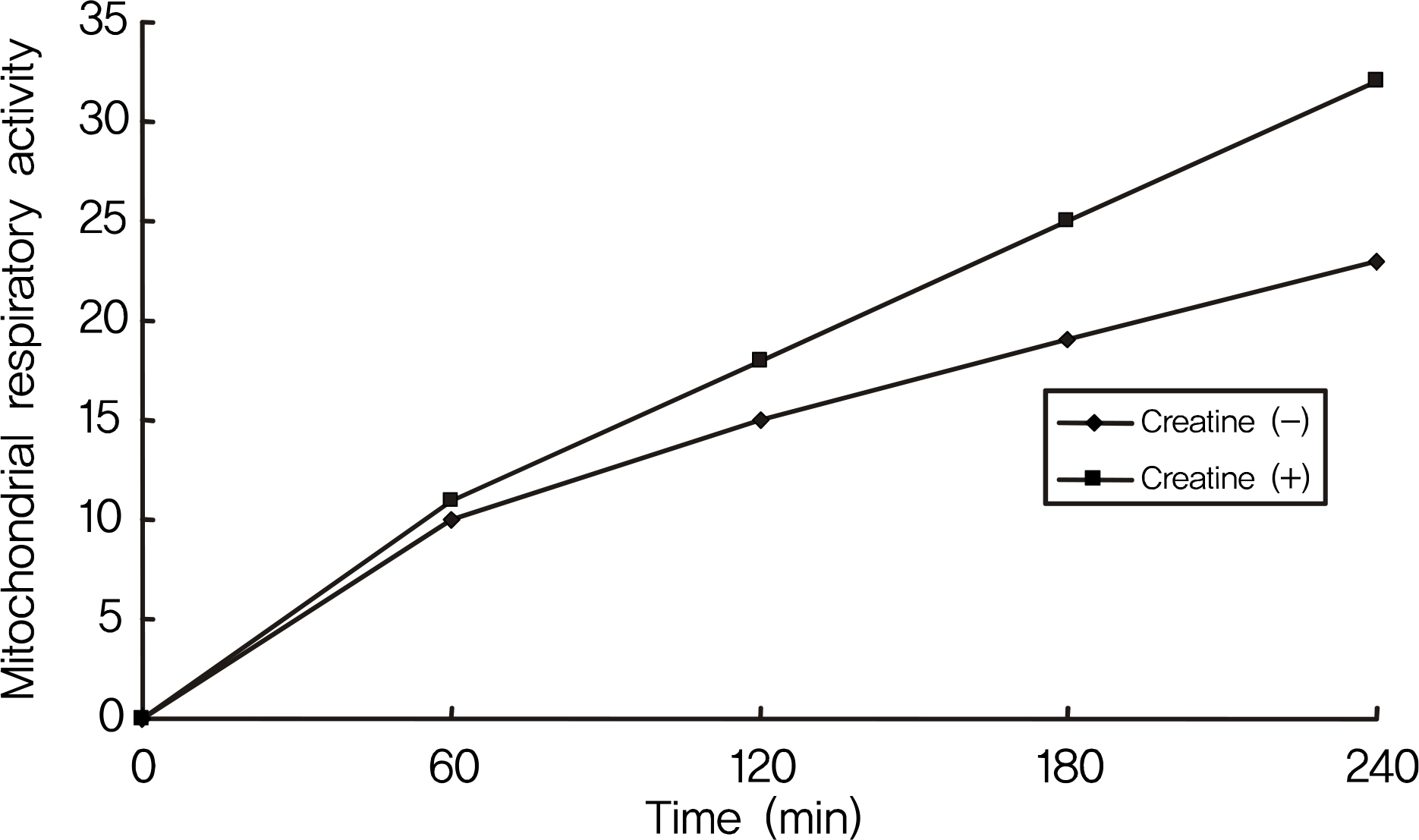
Creatine increased cellular survival of RGC-5 cells significantly after serum deprivation. Additionally, creatine increased mitochondrial respiratory activity and inhibited apoptosis of RGC-5 cells.
CONCLUSIONS
The energy precursor creatine increased survival of retinal ganglion cells after serum deprivation. Creatine could be relevant for the cytoprotection of retinal ganglion cells.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Ames A 3rd. CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000; 34:42–68.
Article2. Holtzman D, Olson JE, Nguyen H, et al. Brain cellular and mitochondrial respiration in media of altered pH. Metab Brain Dis. 1987; 2:127–37.
Article3. Hemmer W, Wallimann T. Functional aspects of creatine kinase in brain. Dev Neurosci. 1993; 15:249–60.
Article4. Wallimann T, Schnyder T, Schlegel J, et al. Subcellular compart-mentation of creatine kinase isoenzymes, regulation of CK and oc-tameric structure of mitochondrial CK: important aspects of the phosphoryl-creatine circuit. Prog Clin Biol Res. 1989; 315:159–76.5. Wallimann T, Wyss M, Brdiczka D, et al. Intracellular compart-mentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit' for cellular energy homeostasis. Biochem J. 1992; 281:21–40.
Article6. McNaught KS, Carrupt PA, Altomare C, et al. Isoquinoline de-rivatives as endogenous neurotoxins in the aetiology of Parkinson's disease. Biochem Pharmacol. 1998; 56:921–33.
Article7. Brewer GJ, Wallimann TW. Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J Neurochem. 2000; 74:1968–78.8. Michaelis T, Wick M, Fujimori H, et al. Proton MRS of oral creatine supplementation in rats. Cerebral metabolite concentrations and ischemic challenge. NMR Biomed. 1999; 12:309–14.
Article9. Wilken B, Ramirez JM, Probst I, et al. Creatine protects the central respiratory network of mammals under anoxic conditions. Pediatr Res. 1998; 43:8–14.
Article10. Wilken B, Ramirez JM, Probst I, et al. Anoxic ATP depletion in neonatal mice brainstem is prevented by creatine supplementation. Arch Arch Dis Child Fetal Neonatal Ed. 2000; 82:F224–7.
Article11. Zhu S, Li M, Figueroa BE, et al. Prophylactic creatine administration mediates neuroprotection in cerebral ischemia in mice. J Neurosci. 2004; 24:5909–12.
Article12. Adcock KH, Nedelcu J, Loenneker T, et al. Neuroprotection of creatine supplementation in neonatal rats with transient cerebral hy-poxia-ischemia. Dev Neurosci. 2002; 24:382–8.
Article13. Chen L, Roberts R, Friedman DL. Expression of brain-type creatine kinase and ubiquitous mitochondrial creatine kinase in the fetal rat brain: evidence for a nuclear energy shuttle. J Comp Neurol. 1995; 363:389–401.
Article14. Hemmer W, Riesinger I, Wallimann T, et al. Brain-type creatine kinase in photoreceptor cell outer segments: role of a phosphocreatine circuit in outer segment energy metabolism and phototransduction. J Cell Sci. 1993; 106:671–83.
Article15. Dolder M, Walzel B, Speer O, et al. Inhibition of the mitochondrial permeability transition by creatine kinase substrates. Requirement for microcompartmentation. J Biol Chem. 2003; 278:17760–6.16. O'Gorman E, Beutner G, Dolder M, et al. The role of creatine kinase in inhibition of mitochondrial permeability transition. FEBS Lett. 1997; 414:253–7.17. Acosta ML, Kalloniatis M, Christie DL. Creatine transporter localization in developing and adult retina: importance of creatine to retinal function. Am J Physiol Cell Physiol. 2005; 289:C1015–23.
Article18. Krishnamoorthy RR, Agarwal P, Prasanna G, et al. Characterization of a transformed rat retinal ganglion cell line. Brain Res Mol Brain Res. 2001; 86:1–12.19. Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989; 119:203–10.
Article20. Mpoke SS, Wolfe J. Differential staining of apoptotic nuclei in liv-ing cells: application to macronuclear elimination in Tetrahymena. J Histochem Cytochem. 1997; 45:675–83.21. Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995; 184:39–51.
Article22. Osborne NN. Pathogenesis of ganglion “cell death” in glaucoma and neuroprotection: focus on ganglion cell axonal mitochondria. Prog Brain Res. 2008; 173:339–52.
Article23. Abu-Amero KK, Bosley TM. Detection of mitochondrial respiratory dysfunction in circulating lymphocytes using resazurin. Arch Pathol Lab Med. 2005; 129:1295–8.
Article24. Wallimann T, Hemmer W. Creatine kinase in non-muscle tissues and cells. Mol Cell Biochem. 1994; 133:193–220.
Article25. Wallimann T, Wyss M, Brdiczka D, et al. Intracellular compart-mentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit' for cellular energy homeostasis. Biochem J. 1992; 281:21–40.
Article26. Whittingham TS, Lipton P. Cerebral synaptic transmission during anoxia is protected by creatine. J Neurochem. 1981; 37:1618–21.
Article27. Carter AJ, Müller RE, Pschorn U, Stransky W. Preincubation with creatine enhances levels of creatine phosphate and prevents anoxic damage in rat hippocampal slices. J Neurochem. 1995; 64:2691–9.
Article28. Chung I, Zhang Y, Eubanks JH, Zhang L. Attenuation of hypoxic current by intracellular applications of ATP regenerating agents in hippocampal CA1 neurons of rat brain slices. Neuroscience. 1998; 86:1101–7.
Article29. Lemasters JJ, Qian T, Bradham CA, et al. Mitochondrial dysfunction in the pathogenesis of necrotic and apoptotic cell death. J Bioenerg Biomembr. 1999; 31:305–19.30. O’Gorman E, Beutner G, Dolder M, et al. The role of creatine kinase in inhibition of mitochondrial permeability transition. FEBS Lett. 1997; 414:253–7.31. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000; 80:1107–213.
Article32. Wyss M, Smeitink J, Wevers RA, Wallimann T. Mitochondrial creatine kinase: a key enzyme of aerobic energy metabolism. Biochim Biophys Acta. 1992; 1102:119–66.
Article33. Passonneau JV, Lowry OH. Metabolite flux in single neurons during ischemia and anesthesia. Curr Probl Clin Biochem. 1971; 3:198–212.34. Stachowiak O, Dolder M, Wallimann T, Richter C. Mitochondrial creatine kinase is a prime target of peroxynitrite-induced mod-ification and inactivation. J Biol Chem. 1998; 273:16694–9.
Article35. Ganapathy PS, Dun Y, Ha Y, et al. Sensitivity of staurosporine-in-duced differentiated RGC-5 cells to homocysteine. Curr Eye Res. 2010; 35:80–90.
Article36. Wood JP, Chidlow G, Tran T, et al. A comparison of differentiation protocols for RGC-5 cells. Invest Ophthalmol Vis Sci. 2010; 51:3774–83.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Anti-vascular Endothelial Growth Factor Antibody on the Survival of Cultured Retinal Ganglion Cells
- The Effect of Melatonin on Retinal Ganglion Cell Survival in Ischemic Retina
- Effect of Atypical Antipsychotics, Risperidone on Serum Creatine Phosphokinase
- Nitric oxide prevents the bovine cerebral endothelial cell death induced by serum-deprivation
- Prognostic Indicators in Tibial Shaft Fractures ; Serum Creatine Kinase Acticity