Korean J Lab Med.
2007 Dec;27(6):451-457. 10.3343/kjlm.2007.27.6.451.
Comparison of VERSANT Hepatitis B Virus DNA 3.0 Assay with Digene Hybrid Capture II Hepatitis B Virus DNA Test in Relation to Clinical Status of Hepatitis B Virus
- Affiliations
-
- 1Department of Laboratory Medicine, Konkuk University College of Medicine, Seoul, Korea.
- 2Department of Laboratory Medicine, Hallym University College of Medicine, Seoul, Korea. dearmina@hanmail.net
- 3Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Laboratory Medicine, Seoul National University Boramae Hospital, Seoul, Korea.
- KMID: 1781550
- DOI: http://doi.org/10.3343/kjlm.2007.27.6.451
Abstract
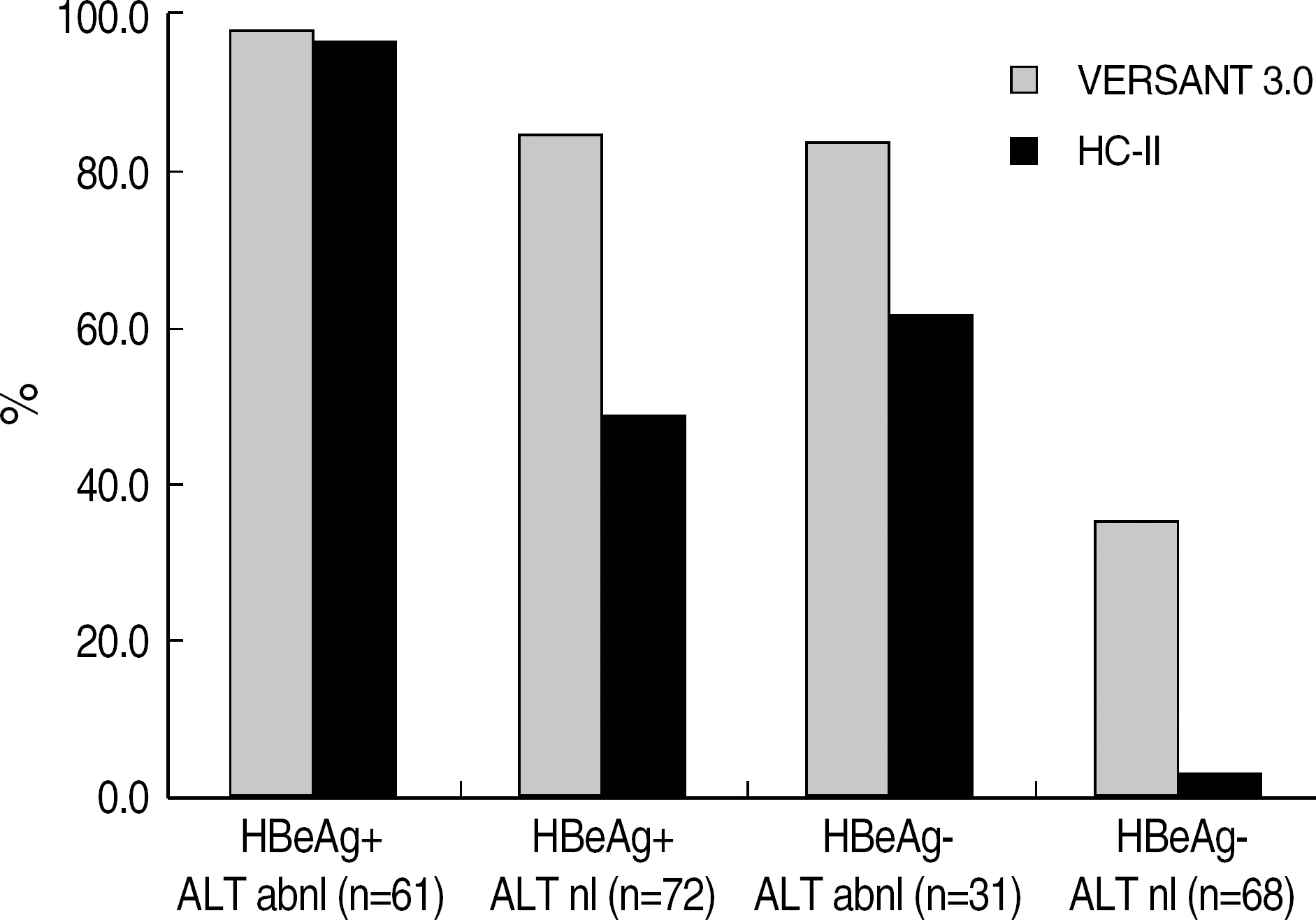
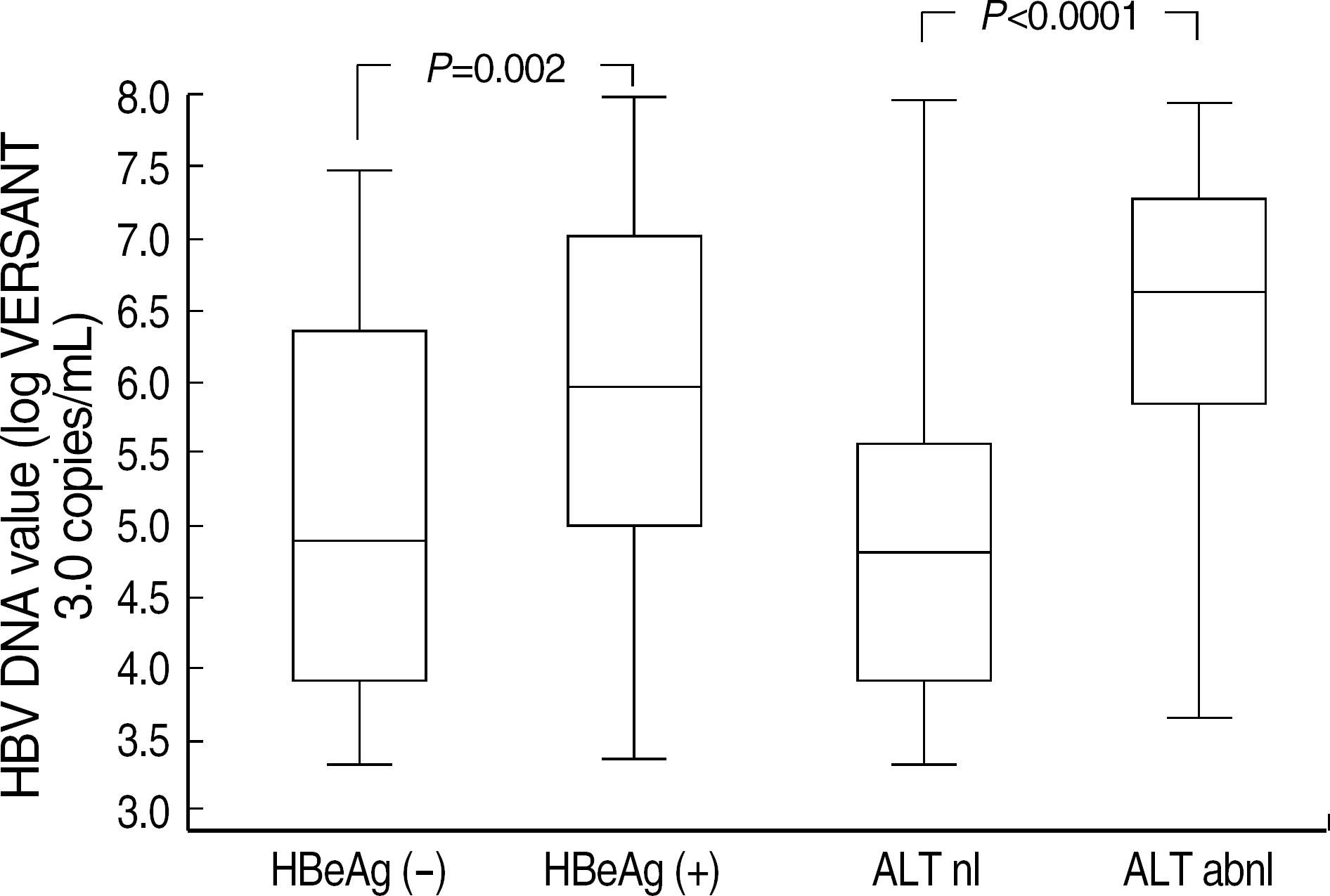
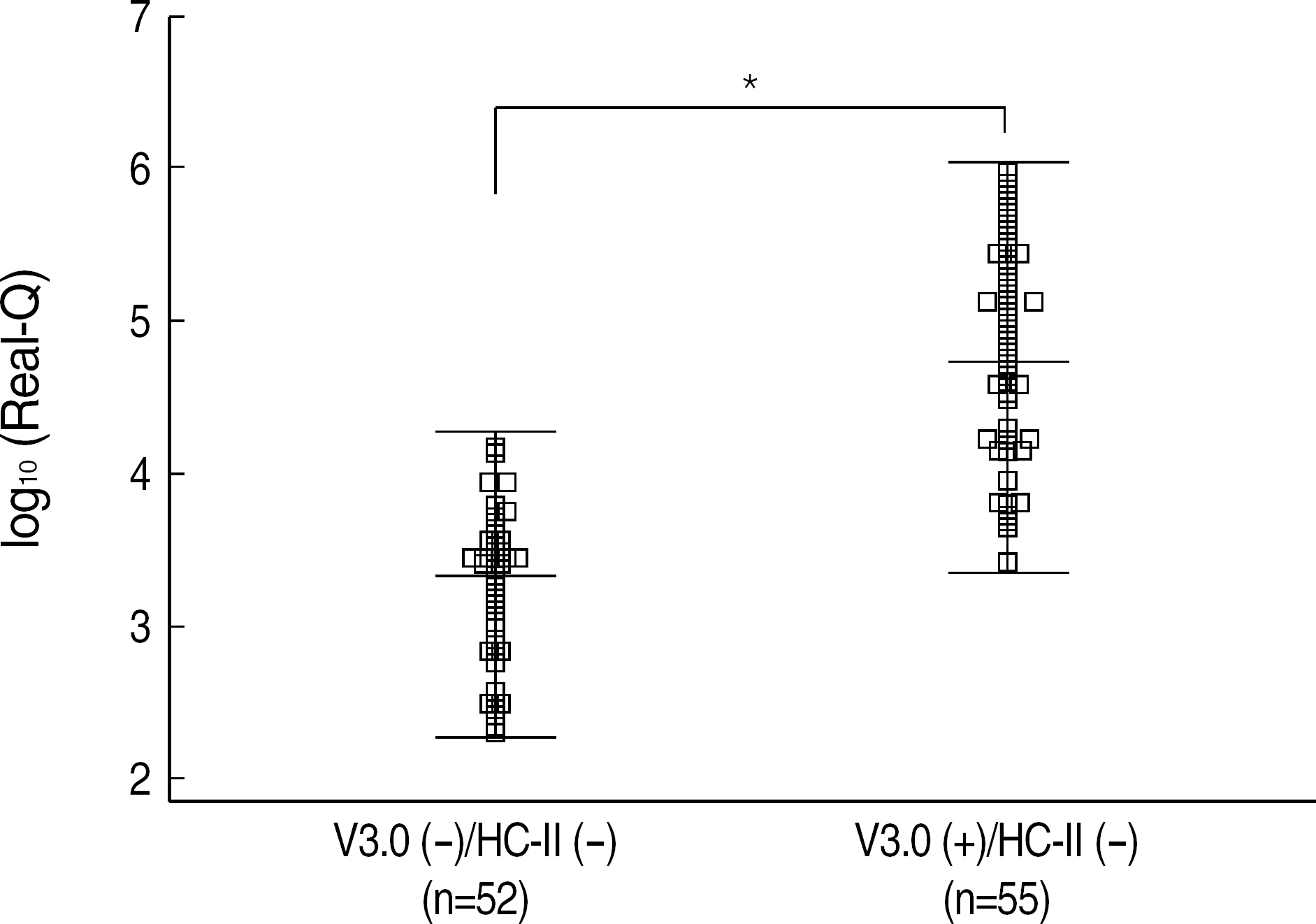
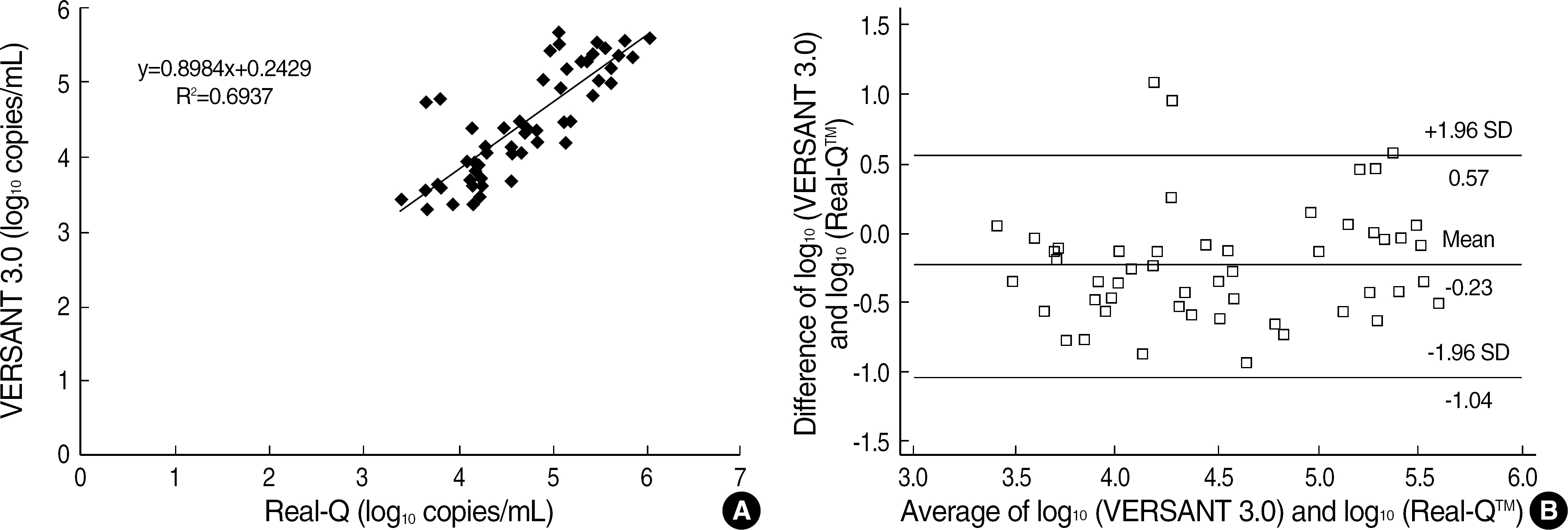
- BACKGROUND: Some differences exist among various Hepatitis B virus (HBV) DNA quantification assays due to lack of standardization and besides clinical usefulness has not been firmly elucidated in Korean HBV patients. METHODS: We compared Bayer VERSANT HBV DNA 3.0 Assay (VERSANT 3.0) with Digene Hybrid Capture II HBV DNA Test (HC-II) according to HBeAg status and ALT levels in 232 HBV-infected Korean patients. One hundred and seventeen sera with undetectable DNA levels by HC-II were further analyzed by Real-Q HBV quantification assay (BioSewoom). RESULTS: Although VERSANT 3.0 and HC-II showed an excellent correlation (r=0.9739), the results (copies/mL) by VERSANT 3.0 were 0.45 log10 higher than those by HC-II. HBV DNA levels were higher in HBeAg-positive group than in HBeAg-negative group (P=0.002), and in abnormal ALT group than in normal ALT group (P<0.0001). The detection rate of HBV DNA by VERSANT 3.0 was lower in HBeAg-negative and normal ALT group (n=68) than in HBeAg-positive or abnormal ALT group (n=164) (35.3% vs 89.6%, P<0.0001). Fifty two sera out of 61 sera with undetectable DNA by VERSANT 3.0 were measurable by Real-Q with mean value of 3.26 log10 copies/mL. CONCLUSIONS: VERSANT 3.0 and HC-II showed an excellent correlation, but a little difference (0.45 log10) existed. VERSANT 3.0 effectively measured clinically relevant HBV DNA levels in most HBVinfected patients in Korea. However, more sensitive assays are needed for patients with negative HBeAg and normal ALT to see the low copies of HBV DNA levels.
MeSH Terms
-
Adolescent
Adult
Aged
Aged, 80 and over
Alanine Transaminase/blood
DNA, Viral/*analysis/genetics
Data Interpretation, Statistical
Female
Hepatitis B e Antigens/metabolism
Hepatitis B virus/genetics/*isolation & purification
Hepatitis B, Chronic/*diagnosis
Humans
Male
Middle Aged
Nucleic Acid Hybridization/*methods
Polymerase Chain Reaction
Regression Analysis
Reproducibility of Results
Sensitivity and Specificity
Figure
Reference
-
1.Jardi R., Buti M., Rodriguez-Frias F., Cortina M., Esteban R., Guardia J, et al. The value of quantitative detection of HBV-DNA amplified by PCR in the study of hepatitis B infection. J Hepatol. 1996. 24:680–5.2.Ho SK., Chan TM. An overview of assays for serum HBV DNA. Clin Lab. 2000. 46:609–14.3.Zeuzem S. Overview of commercial HBV assays systems. Methods Mol Med. 2004. 95:3–13.4.Ho SK., Chan TM., Cheng IK., Lai KN. Comparison of the second-generation digene hybrid capture assay with the branched-DNA assay for measurement of hepatitis B virus DNA in serum. J Clin Microbiol. 1999. 37:2461–5.
Article5.Yao JD., Beld MG., Oon LL., Sherlock CH., Germer J., Menting S, et al. Multicenter evaluation of the VERSANT hepatitis B virus DNA 3.0 assay. J Clin Microbiol. 2004. 42:800–6.
Article6.Lai CL., Chien RN., Leung NW., Chang TT., Guan R., Tai DI, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998. 339:61–8.7.Puchhammer-Stockl E., Mandl CW., Kletzmayr J., Holzmann H., Hofmann A., Aberle SW, et al. Monitoring the virus load can predict the emergence of drug-resistant hepatitis B virus strains in renal transplantation patients during lamivudine therapy. J Infect Dis. 2000. 181:2063–6.
Article8.Clinical and Laboratory Standards Institute. User verification of performance for precision and trueness; approved guideline-second edition, CLSI document EP15-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2005.9.Clinical and Laboratory Standards Institute. Evaluation of precision performance of quantitative measurement methods; approved guideline-second edition, CLSI document EP5-A2. 2004. Wayne, PA: Clinical and Laboratory Standards Institute;2004.10.Bland JM., Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995. 346:1085–7.
Article11.Pawlotsky JM., Bastie A., Hezode C., Lonjon I., Darthuy F., Remire J, et al. Routine detection and quantification of hepatitis B virus DNA in clinical laboratories: performance of three commercial assays. J Virol Methods. 2000. 85:11–21.
Article12.Chan HL., Leung NW., Lau TC., Wong ML., Sung JJ. Comparison of three different sensitive assays for hepatitis B virus DNA in monitoring of responses to antiviral therapy. J Clin Microbiol. 2000. 38:3205–8.
Article13.Gilbert N., Corden S., Ijaz S., Grant PR., Tedder RS., Boxall EH. Comparison of commercial assays for the quantification of HBV DNA load in health care workers: calibration differences. J Virol Methods. 2002. 100:37–47.
Article14.Dai CY., Yu ML., Chen SC., Lin ZY., Hsieh MY., Wang LY, et al. Clinical evaluation of the COBAS Amplicor HBV monitor test for measuring serum HBV DNA and comparison with the Quantiplex branched DNA signal amplification assay in Taiwan. J Clin Pathol. 2004. 57:141–5.
Article15.Ronsin C., Pillet A., Bali C., Denoyel GA. Evaluation of the COBAS AmpliPrep-total nucleic acid isolation-COBAS TaqMan hepatitis B virus (HBV) quantitative test and comparison to the VERSANT HBV DNA 3.0 assay. J Clin Microbiol. 2006. 44:1390–9.
Article16.Heo J., Cho M., Cho BM., Lee SM., Kim TO., Kim GH, et al. Predictors of lamivudine resistance in patients with chronic hepatitis B virus infection. Korean J Hepatol. 2007. 13:157–65. (허정, 조몽, 조병만, 이선미, 김태오, 김광하등. 만성 B형간질환환자의라미부딘내성예측인자. 대한간학회지 2007;13: 157-65.).17.Byun KS., Lee KS., Cho M., Suh KS., Kweon Y., Koh KC, et al. Guideline for the treatment of chronic hepatitis B virus infection. Korean J Hepatol. 2004. 10(S):S89–100. (변관수, 이관식, 조몽, 서경석, 권오건, 고광철등. 2004년대한간학회 B형만성간염치료가이드라인. 대한간학회지 2004;. 10:S89-100.).18.Welzel TM., Miley WJ., Parks TL., Goedert JJ., Whitby D., Ortiz-Conde BA. Real-time PCR assay for detection and quantification of hepatitis B virus genotypes A to G. J Clin Microbiol. 2006. 44:3325–33.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevention of Viral Hepatitis and Vaccination
- Comparison of Hepatitis B Virus Detection by Direct Hybridization Assay and Polymerase Chain Reaction
- Comparison of Hybrid Capture System, Hybrid Capture II and Quantiplex HBV DNA Assay for Quantitation of Hepatitis B Virus DNA
- Comparison of the Hybrid Capture Assay and Polymerase Chain Reaction for the Detection of Hepatitis B Virus DNA
- Relative etiology role of hepatitis B virus and hepatitis C virus in HBsAg-negative patients with chronic liver disease in Korea: determination of serum HBV DNA using polymerase chain reaction and of srum anti-HCV using ELISA