Korean J Lab Med.
2007 Oct;27(5):305-312. 10.3343/kjlm.2007.27.5.305.
Clinical Significance of Quantitation of WT1 Gene Expression for Minimal Residual Disease Monitoring of Acute Myelogenous Leukemia
- Affiliations
-
- 1Department of Laboratory Medicine, Busan Paik Hospital, College of Medicine, Inje University, Busan, Korea. jeong418@medimail.co.kr
- 2Paik Institute for Clinical Research, Inje University, Busan, Korea.
- 3Department of Laboratory Medicine, Pusan National University School of Medicine, Busan, Korea.
- KMID: 1781527
- DOI: http://doi.org/10.3343/kjlm.2007.27.5.305
Abstract
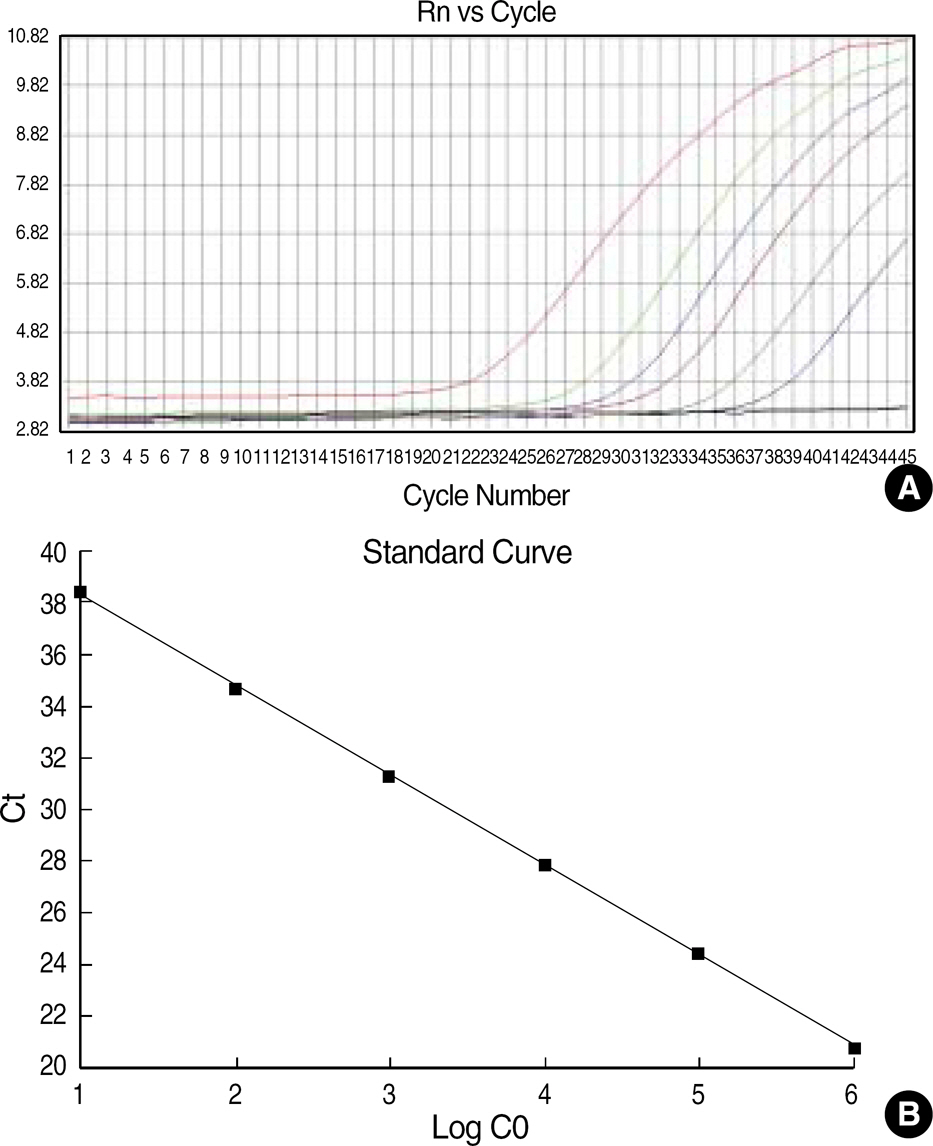
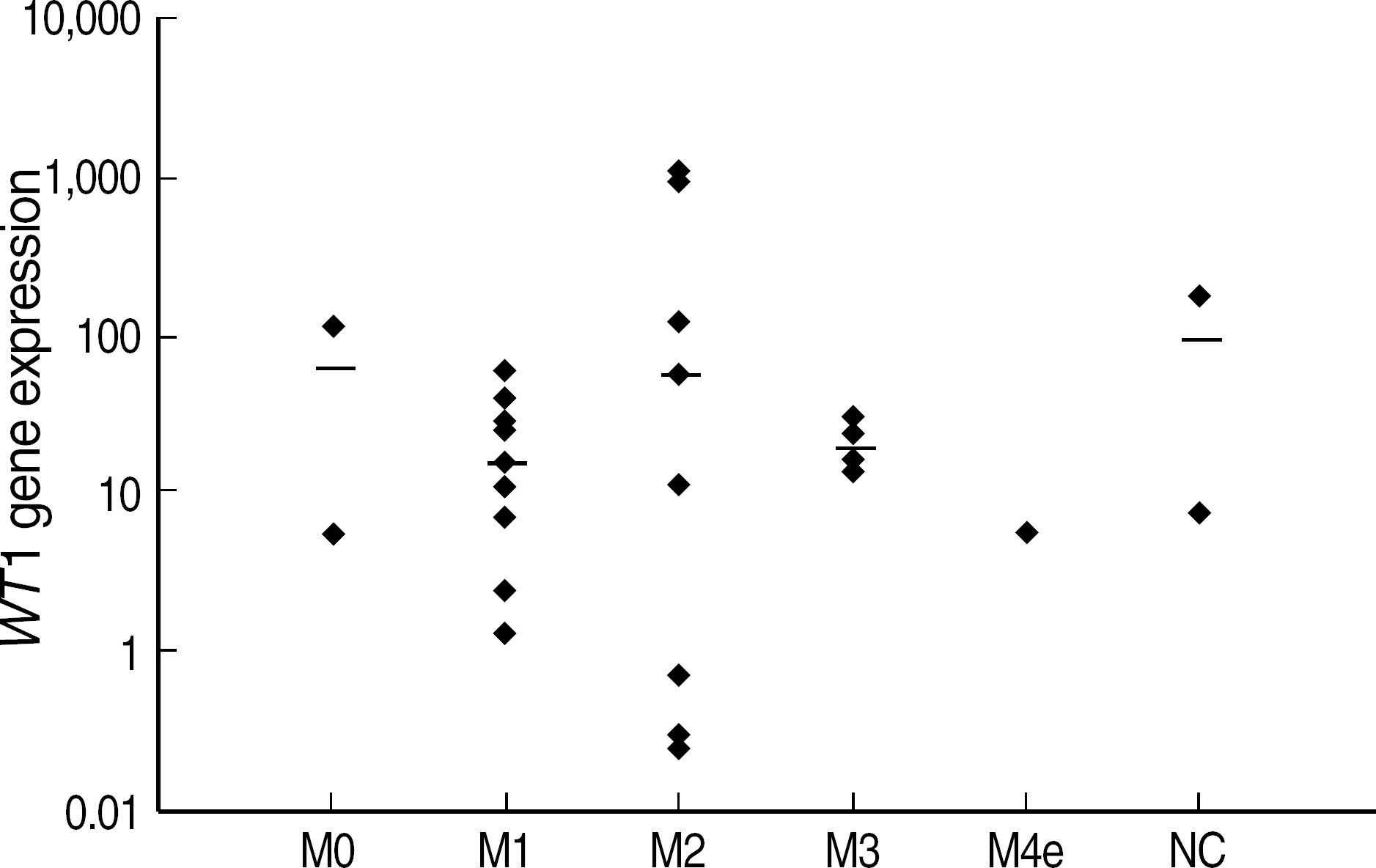
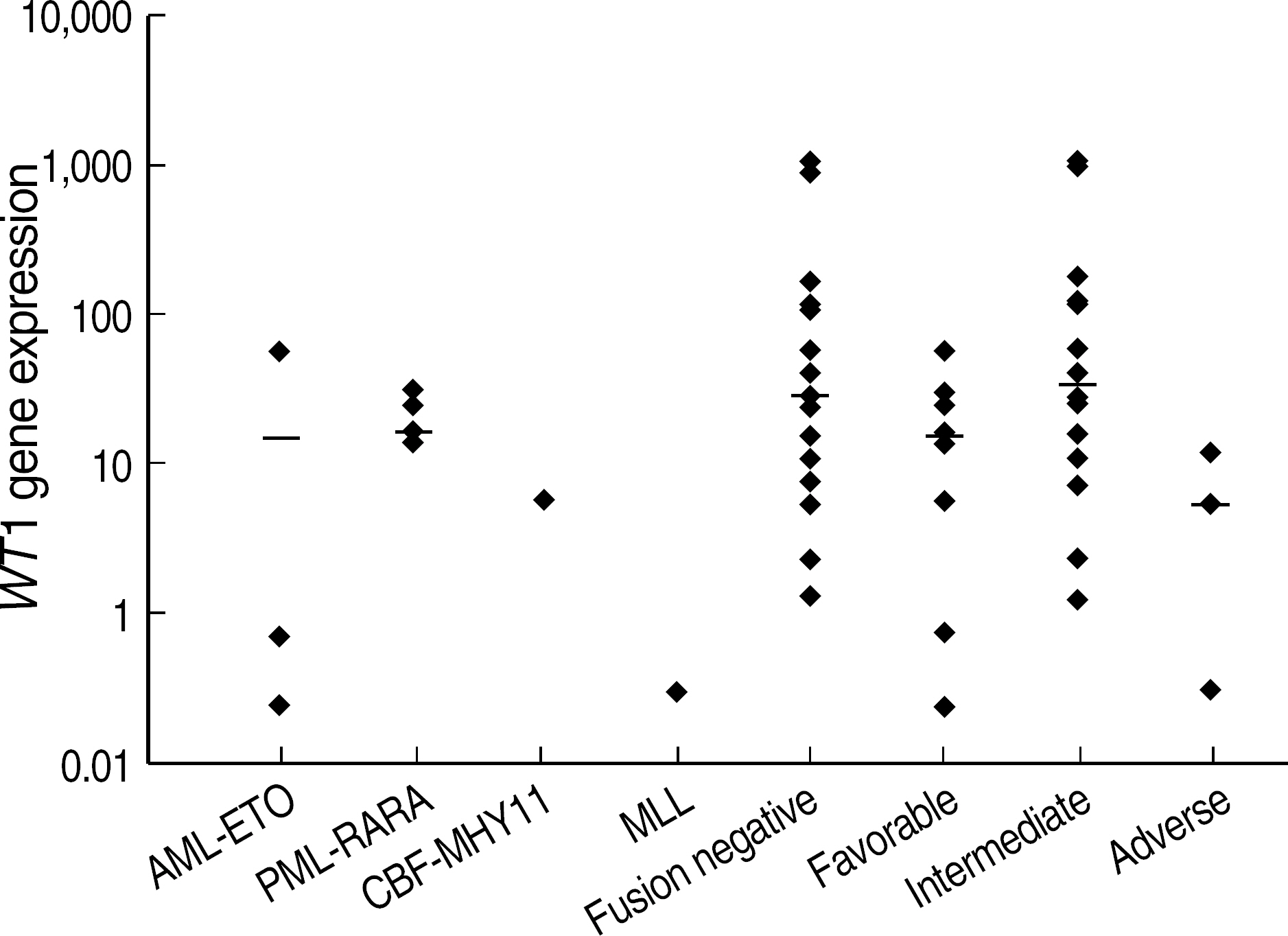
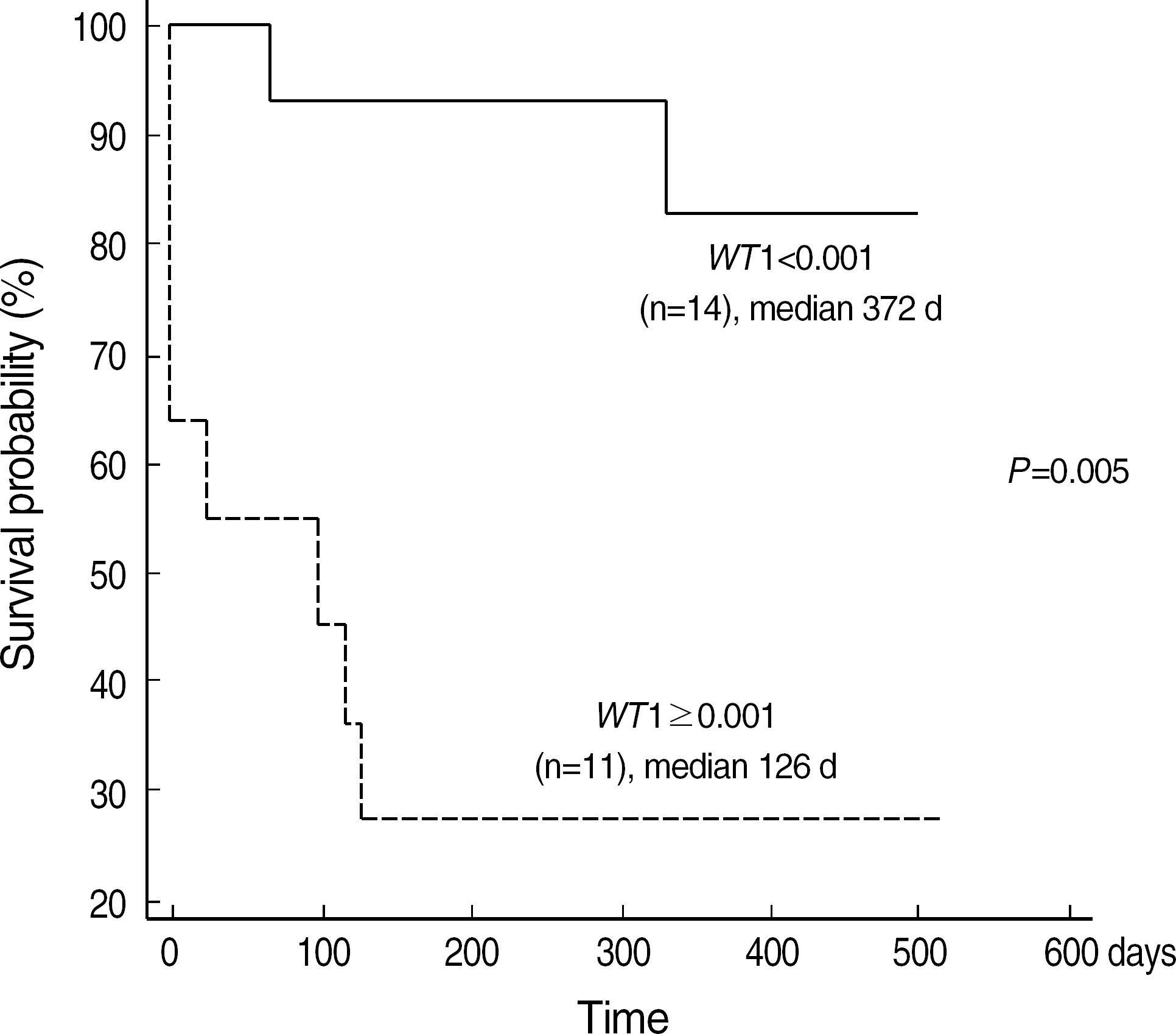
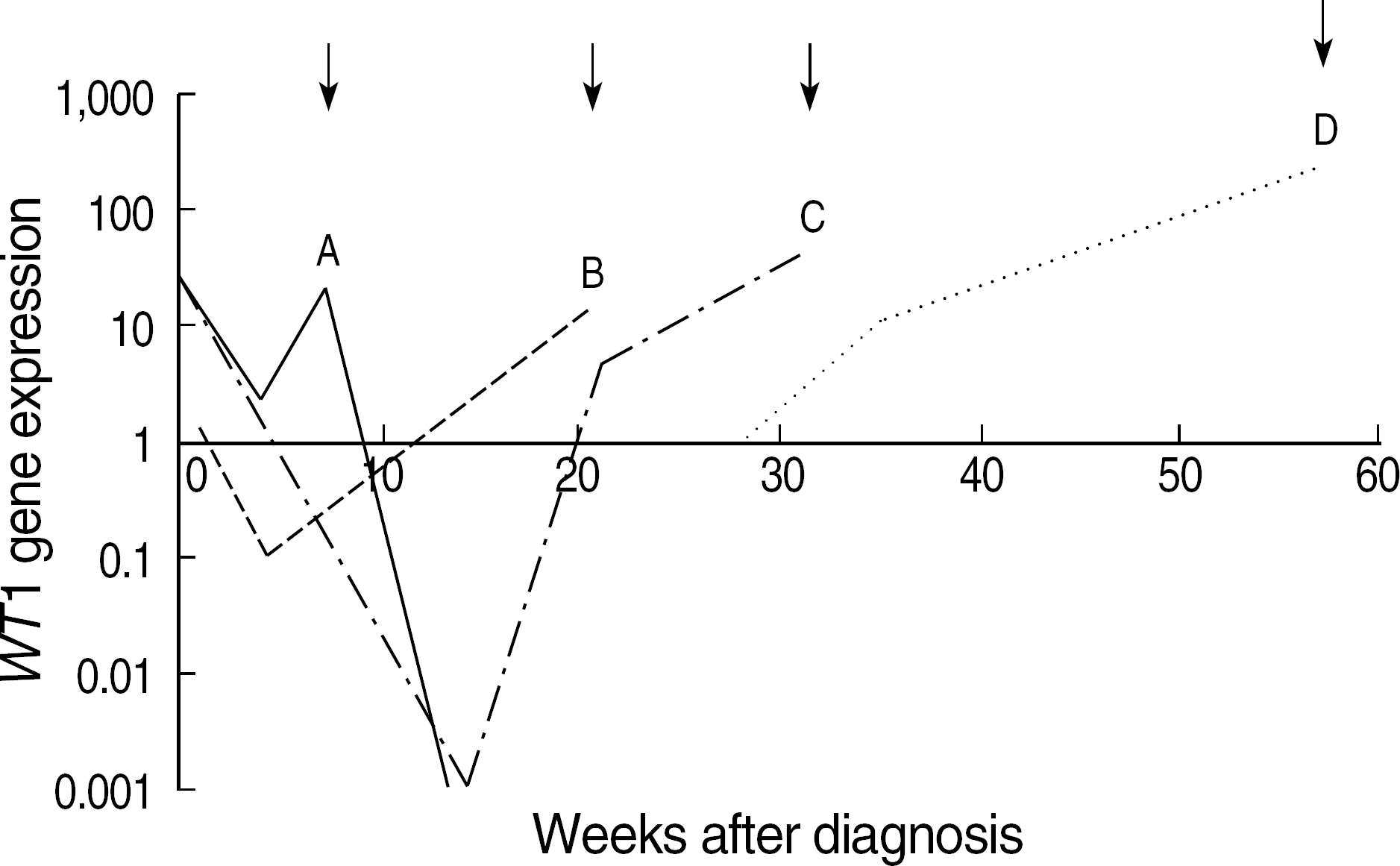
- BACKGROUND: Following induction chemotherapy for AML, a sensitive determination of minimal residual disease (MRD) in patients achieving complete remission (CR) should enable the detection of early relapse. This study was designed to verify if quantitative assessment of the Wilms' tumor (WT1) gene by real time polymerase chain reaction (RQ-PCR) can be used as a marker for MRD detection during the monitoring of AML. METHODS: WT1 gene expression was quantified by RQ-PCR in 31 patients with AML at diagnosis (27 patients) and during follow-up (29 patients) relative to ABL control gene. In four patients, the WT1 gene expression was analyzed in comparison to a second PCR marker, PML-RARA fusion transcript. Prognostic significance of WT1 gene expression was analyzed at diagnosis and at the primary CR evaluation. Longitudinal WT1 gene analysis was performed in 17 AML patients. RESULTS: At diagnosis, WT1 gene expression exceeded the control level in all of the patients. Higher levels of WT1 gene expression were not associated with shorter event free survival or overall survival at diagnosis. Higher levels of WT1 gene expression were associated with shorter event free survival after induction chemotherapy. Relapse was observed in eight of 17 patients analysed longitudinally, and an increase of WT1 gene expression preceded morphologic relapse in four patients with the fusion transcript negative. Concomitant monitoring of PML-RARA fusion transcript reveals the lack of a significant correlation withWT1 gene expression. CONCLUSIONS: Quantitation of WT1 gene expression could be used for MRD monitoring of AML and for the early detection of relapse, especially in patients lacking specific molecular markers.
Keyword
MeSH Terms
-
Adaptor Proteins, Signal Transducing/analysis
Adult
Aged
Aged, 80 and over
Female
Follow-Up Studies
Gene Expression
*Genes, Wilms Tumor
Humans
Leukemia, Myelomonocytic, Acute/*diagnosis/mortality/therapy
Male
Middle Aged
Neoplasm, Residual
Polymerase Chain Reaction
Prognosis
Survival Analysis
WT1 Proteins/*analysis/genetics
Figure
Reference
-
1.Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002. 29:23–39.
Article2.van der Velden VH., Hochhaus A., Cazzaniga G., Szczepanski T., Gabert J., van Dongen JJ. Detection of minimal residual disease in hematologic malignancies by real-time quantitative PCR: principles, approaches, and laboratory aspects. Leukemia. 2003. 17:1013–34.
Article3.Ostergaard M., Olesen LH., Hasle H., Kjeldsen E., Hokland P. WT1 gene expression: an excellent tool for monitoring minimal residual disease in 70% of acute myeloid leukaemia patients-results from a single-centre study. Br J Haematol. 2004. 125:590–600.4.Lin F., van Rhee F., Goldman JM., Cross NC. Kinetics of increasing BCR-ABL transcript numbers in chronic myeloid leukemia patients who relapse after bone marrow transplantation. Blood. 1996. 87:4473–8.5.Olavarria E., Kanfer E., Szydlo R., Kaeda J., Rezvani K., Cwynarski K, et al. Early detection of BCR-ABL transcripts by quantitative reverse transcriptase-polymerase chain reaction predicts outcome after allogeneic stem cell transplantation for chronic myeloid leukemia. Blood. 2001. 97:1560–5.6.Ogawa H., Tamaki H., Ikegame K., Soma T., Kawakami M., Tsuboi A, et al. The usefulness of monitoring WT1 gene transcripts for the prediction and management of relapse following allogeneic stem cell transplantation in acute type leukemia. Blood. 2003. 101:1698–704.7.Pallisgaard N., Hokland P., Riishoj DC., Pedersen B., Jorgensen P. Multiplex reverse transcription-polymerase chain reaction for simultaneous screening of 29 translocations and chromosomal aberrations in acute leukemia. Blood. 1998. 92:574–88.
Article8.Inoue K., Sugiyama H., Ogawa H., Nakagawa M., Yamagami T., Miwa H, et al. WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood. 1994. 84:3071–9.9.Inoue K., Ogawa H., Sonoda Y., Kimura T., Sakabe H., Oka Y, et al. Aberrant overexpression of the Wilms tumor gene (WT1) in human leukemia. Blood. 1997. 89:1405–12.10.Miwa H., Beran M., Saunders GF. Expression of the Wilms' tumor gene (WT1) in human leukemias. Leukemia. 1992. 6:405–9.11.Miyagi T., Ahuja H., Kudota T. Expression of the candidate Wilms' tumor gene, WT1, in human leukemia cells. Leukemia. 1993. 7:970–7.12.Brieger J., Weidmann E., Fenchel K., Mitrou PS., Hoelzer D., Bergmann L. The expression of the Wilms' tumor gene in acute myelocytic leukemias as a possible marker for leukemic blast cells. Leukemia. 1994. 8:2138–43.13.Menssen HD., Renkl HJ., Rodeck U., Maurer J., Notter M., Schwartz S, et al. Presence of Wilms' tumor gene (WT1) transcripts and the WT1 nuclear protein in the majority of human acute leukemias. Leukemia. 1995. 9:1060–7.14.Gessler M., Poustka A., Cavenee W., Neve RL., Orkin SH., Bruns GA. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990. 343:774–8.
Article15.Maurer U., Brieger J., Weidmann E., Mitrou PS., Hoelzer D., Bergmann L. The Wilms' tumor gene is expressed in a subset of CD34+ progenitors and downregulated early in the course of differentiation in vitro. Exp Hematol. 1997. 25:945–50.16.Algar EM., Khromykh T., Smith SI., Blackburn DM., Bryson GJ., Smith PJ. A WT1 antisense oligonucleotide inhibits proliferation and induces apoptosis in myeloid leukaemia cell lines. Oncogene. 1996. 12:1005–14.17.Svedberg H., Chylicki K., Baldetorp B., Rauscher FJ 3rd., Gullberg U. Constitutive expression of the Wilms' tumor gene (WT1) in the leukemic cell line U937 blocks parts of the differentiation program. Oncogene. 1998. 16:925–32.18.Schmid D., Heinze G., Linnerth B., Tisljar K., Kusec R., Geissler K, et al. Prognostic significance of WT1 gene expression at diagnosis in adult de novo acute myeloid leukemia. Leukemia. 1997. 11:639–43.19.Garg M., Moore H., Tobal K., Liu Yin JA. Prognostic significance of quantitative analysis of WT 1 gene transcripts by competitive reverse transcription polymerase chain reaction in acute leukaemia. Br J Haematol. 2003. 123:49–59.20.Gaiger A., Linnerth B., Mann G., Schmid D., Heinze G., Tisljar K, et al. Wilms' tumour gene (WT1) expression at diagnosis has no prognostic relevance in childhood acute lymphoblastic leukaemia treated by an intensive chemotherapy protocol. Eur J Haematol. 1999. 63:86–93.21.Bergmann L., Miething C., Maurer U., Brieger J., Karakas T., Weidmann E, et al. High levels of Wilms' tumor gene (WT1) mRNA in acute myeloid leukemias are associated with a worse long-term outcome. Blood. 1997. 90:1217–25.22.Grimwade D., Walker H., Oliver F., Wheatley K., Harrison C., Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998. 92:2322–33.23.Gabert J., Beillard E., van der Velden VH., Bi W., Grimwade D., Pallisgaard N, et al. Standardization and quality control studies of ‘realtime’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia – a Europe Against Cancer Program. Leukemia. 2003. 17:2318–57.
Article24.Weissor M., Kern W., Rauhut S., Schoch C., Hiddemann W., Haferlach T, et al. Prognostic impact of RT-PCR-based quantification of WT1 gene expression during MRD monitoring of acute myeloid leukemia. Leukemia. 2005. 19:1416–23.25.Cassinat B., Zassadowski F., Balitrand N., Barbay C., Rain JD., Fenaux P, et al. Quantitation of minimal residual disease in acute promyelocytic leukemia patients with t(15;17) translocation using real-time RT-PCR. Leukemia. 2000. 14:324–8.
Article26.Cilloni D., Saglio G. Usefulness of quantitative assessment of Wilms tumor suppressor gene expression in chronic myeloid leukemia patients undergoing imatinib therapy. Semin Hematol. 2003. 40:37–41.
Article27.Spinsanti P., de Grazia U., Faggioni A., Frati L., Calogero A., Ragona G. Wilms' tumor gene expression by normal and malignant human B lymphocytes. Leuk Lymphoma. 2000. 38:611–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of WT1 Gene in Childhood Acute Leukemia
- Monitoring of WT-1 gene expression in peripheral blood of patients with acute leukemia by semiquantitative RT-PCR; possible marker for detection of minimal residual leukemia
- Prognostic Significance of WT1 Gene Expression in Patients with Acute Leukemia
- Measurements of treatment response in childhood acute leukemia
- Sweet's Syndrome with Myelodysplastic Syndrome Progressing to Acute Myelogenous Leukemia