Korean J Hematol.
2012 Dec;47(4):245-254. 10.5045/kjh.2012.47.4.245.
Measurements of treatment response in childhood acute leukemia
- Affiliations
-
- 1Department of Paediatrics, Yong Loo Lin School of Medicine, National University of Singapore, Singapore. paedc@nus.edu.sg
- KMID: 1832102
- DOI: http://doi.org/10.5045/kjh.2012.47.4.245
Abstract
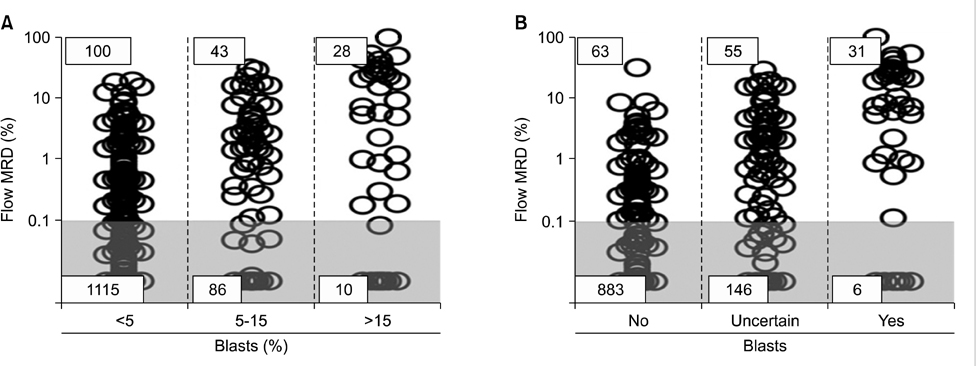
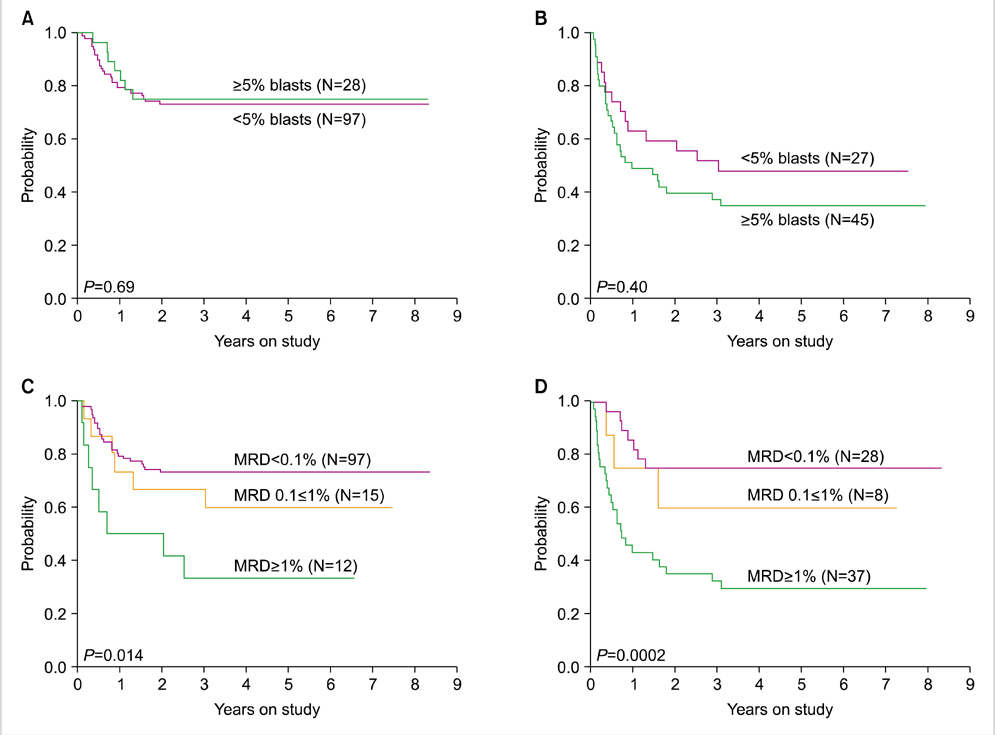
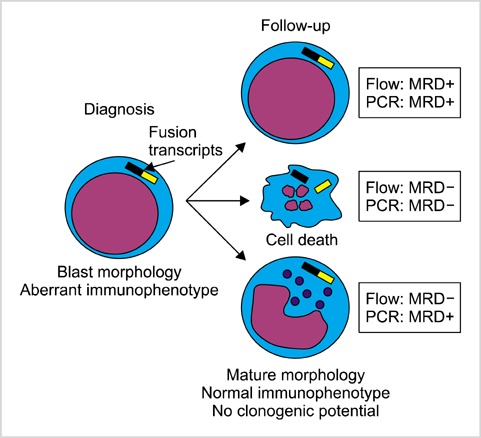
- Measuring response to chemotherapy is a backbone of the clinical management of patients with acute leukemia. This task has historically relied on the ability to identify leukemic cells among normal bone marrow cells by their morphology. However, more accurate ways to identify leukemic cells have been developed, which allow their detection even when they are present in small numbers that would be impossible to be recognized by microscopic inspection. The levels of such minimal residual disease (MRD) are now widely used as parameters for risk assignment in acute lymphoblastic leukemia (ALL) and increasingly so in acute myeloid leukemia (AML). However, different MRD monitoring methods may produce discrepant results. Moreover, results of morphologic examination may be in stark contradiction to MRD measurements, thus creating confusion and complicating treatment decisions. This review focusses on the relation between results of different approaches to measure response to treatment and define relapse in childhood acute leukemia.
Keyword
MeSH Terms
Figure
Reference
-
1. Campana D, Pui CH. Detection of minimal residual disease in acute leukemia: methodologic advances and clinical significance. Blood. 1995. 85:1416–1434.
Article2. Bradstock KF, Janossy G, Tidman N, et al. Immunological monitoring of residual disease in treated thymic acute lymphoblastic leukaemia. Leuk Res. 1981. 5:301–309.
Article3. Campana D. Minimal residual disease in acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2010. 2010:7–12.
Article4. Brüggemann M, Schrauder A, Raff T, et al. Standardized MRD quantification in European ALL trials: proceedings of the Second International Symposium on MRD assessment in Kiel, Germany, 18-20 September 2008. Leukemia. 2010. 24:521–535.
Article5. Shook D, Coustan-Smith E, Ribeiro RC, Rubnitz JE, Campana D. Minimal residual disease quantitation in acute myeloid leukemia. Clin Lymphoma Myeloma. 2009. 9:Suppl 3. S281–S285.
Article6. Buccisano F, Maurillo L, Del Principe MI, et al. Prognostic and therapeutic implications of minimal residual disease detection in acute myeloid leukemia. Blood. 2012. 119:332–341.
Article7. Brisco MJ, Condon J, Hughes E, et al. Outcome prediction in childhood acute lymphoblastic leukaemia by molecular quantification of residual disease at the end of induction. Lancet. 1994. 343:196–200.
Article8. Cavé H, van der Werff ten Bosch J, Suciu S, et al. European Organization for Research and Treatment of Cancer-Childhood Leukemia Cooperative Group. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. N Engl J Med. 1998. 339:591–598.
Article9. Coustan-Smith E, Behm FG, Sanchez J, et al. Immunological detection of minimal residual disease in children with acute lymphoblastic leukaemia. Lancet. 1998. 351:550–554.
Article10. van Dongen JJ, Seriu T, Panzer-Grümayer ER, et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998. 352:1731–1738.
Article11. Coustan-Smith E, Sancho J, Hancock ML, et al. Clinical importance of minimal residual disease in childhood acute lymphoblastic leukemia. Blood. 2000. 96:2691–2696.
Article12. Coustan-Smith E, Sancho J, Behm FG, et al. Prognostic importance of measuring early clearance of leukemic cells by flow cytometry in childhood acute lymphoblastic leukemia. Blood. 2002. 100:52–58.
Article13. Coustan-Smith E, Sancho J, Hancock ML, et al. Use of peripheral blood instead of bone marrow to monitor residual disease in children with acute lymphoblastic leukemia. Blood. 2002. 100:2399–2402.
Article14. Nyvold C, Madsen HO, Ryder LP, et al. Precise quantification of minimal residual disease at day 29 allows identification of children with acute lymphoblastic leukemia and an excellent outcome. Blood. 2002. 99:1253–1258.
Article15. Dworzak MN, Fröschl G, Printz D, et al. Prognostic significance and modalities of flow cytometric minimal residual disease detection in childhood acute lymphoblastic leukemia. Blood. 2002. 99:1952–1958.
Article16. Zhou J, Goldwasser MA, Li A, et al. Quantitative analysis of minimal residual disease predicts relapse in children with B-lineage acute lymphoblastic leukemia in DFCI ALL Consortium Protocol 95-01. Blood. 2007. 110:1607–1611.
Article17. Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood. 2008. 111:5477–5485.
Article18. Basso G, Veltroni M, Valsecchi MG, et al. Risk of relapse of childhood acute lymphoblastic leukemia is predicted by flow cytometric measurement of residual disease on day 15 bone marrow. J Clin Oncol. 2009. 27:5168–5174.
Article19. Sutton R, Venn NC, Tolisano J, et al. Clinical significance of minimal residual disease at day 15 and at the end of therapy in childhood acute lymphoblastic leukaemia. Br J Haematol. 2009. 146:292–299.
Article20. Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010. 115:3206–3214.21. Stow P, Key L, Chen X, et al. Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia. Blood. 2010. 115:4657–4663.
Article22. Yamaji K, Okamoto T, Yokota S, et al. Minimal residual disease-based augmented therapy in childhood acute lymphoblastic leukemia: a report from the Japanese Childhood Cancer and Leukemia Study Group. Pediatr Blood Cancer. 2010. 55:1287–1295.
Article23. Katsibardi K, Moschovi MA, Braoudaki M, Papadhimitriou SI, Papathanasiou C, Tzortzatou-Stathopoulou F. Sequential monitoring of minimal residual disease in acute lymphoblastic leukemia: 7-year experience in a pediatric hematology/oncology unit. Leuk Lymphoma. 2010. 51:846–852.
Article24. Meleshko AN, Savva NN, Fedasenka UU, et al. Prognostic value of MRD-dynamics in childhood acute lymphoblastic leukemia treated according to the MB-2002/2008 protocols. Leuk Res. 2011. 35:1312–1320.
Article25. Eckert C, Biondi A, Seeger K, et al. Prognostic value of minimal residual disease in relapsed childhood acute lymphoblastic leukaemia. Lancet. 2001. 358:1239–1241.
Article26. Coustan-Smith E, Gajjar A, Hijiya N, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia after first relapse. Leukemia. 2004. 18:499–504.
Article27. Paganin M, Zecca M, Fabbri G, et al. Minimal residual disease is an important predictive factor of outcome in children with relapsed 'high-risk' acute lymphoblastic leukemia. Leukemia. 2008. 22:2193–2200.
Article28. Raetz EA, Borowitz MJ, Devidas M, et al. Reinduction platform for children with first marrow relapse of acute lymphoblastic leukemia: A Children's Oncology Group Study. J Clin Oncol. 2008. 26:3971–3978.
Article29. Krejci O, van der Velden VH, Bader P, et al. Level of minimal residual disease prior to haematopoietic stem cell transplantation predicts prognosis in paediatric patients with acute lymphoblastic leukaemia: a report of the Pre-BMT MRD Study Group. Bone Marrow Transplant. 2003. 32:849–851.
Article30. Bader P, Kreyenberg H, Henze GH, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009. 27:377–384.
Article31. Leung W, Campana D, Yang J, et al. High success rate of hematopoietic cell transplantation regardless of donor source in children with very high-risk leukemia. Blood. 2011. 118:223–230.
Article32. Zhao XS, Liu YR, Zhu HH, et al. Monitoring MRD with flow cytometry: an effective method to predict relapse for ALL patients after allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2012. 91:183–192.
Article33. Brüggemann M, Raff T, Flohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006. 107:1116–1123.
Article34. Raff T, Gökbuget N, Lüschen S, et al. Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: data from the GMALL 06/99 and 07/03 trials. Blood. 2007. 109:910–915.
Article35. Holowiecki J, Krawczyk-Kulis M, Giebel S, et al. Status of minimal residual disease after induction predicts outcome in both standard and high-risk Ph-negative adult acute lymphoblastic leukaemia. The Polish Adult Leukemia Group ALL 4-2002 MRD Study. Br J Haematol. 2008. 142:227–237.
Article36. Bassan R, Spinelli O, Oldani E, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood. 2009. 113:4153–4162.
Article37. Patel B, Rai L, Buck G, et al. Minimal residual disease is a significant predictor of treatment failure in non T-lineage adult acute lymphoblastic leukaemia: final results of the international trial UKALL XII/ECOG2993. Br J Haematol. 2010. 148:80–89.
Article38. Gökbuget N, Kneba M, Raff T, et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012. 120:1868–1876.
Article39. Guerrasio A, Rosso C, Martinelli G, et al. Polyclonal haemopoieses associated with long-term persistence of the AML1-ETO transcript in patients with FAB M2 acute myeloid leukaemia in continous clinical remission. Br J Haematol. 1995. 90:364–368.
Article40. Marcucci G, Livak KJ, Bi W, Strout MP, Bloomfield CD, Caligiuri MA. Detection of minimal residual disease in patients with AML1/ETO-associated acute myeloid leukemia using a novel quantitative reverse transcription polymerase chain reaction assay. Leukemia. 1998. 12:1482–1489.
Article41. Tobal K, Newton J, Macheta M, et al. Molecular quantitation of minimal residual disease in acute myeloid leukemia with t(8;21) can identify patients in durable remission and predict clinical relapse. Blood. 2000. 95:815–819.
Article42. Sievers EL, Lange BJ, Alonzo TA, et al. Immunophenotypic evidence of leukemia after induction therapy predicts relapse: results from a prospective Children's Cancer Group study of 252 patients with acute myeloid leukemia. Blood. 2003. 101:3398–3406.
Article43. Coustan-Smith E, Ribeiro RC, Rubnitz JE, et al. Clinical significance of residual disease during treatment in childhood acute myeloid leukaemia. Br J Haematol. 2003. 123:243–252.
Article44. San-Miguel JF, Vidriales MB, Orfão A. Immunological evaluation of minimal residual disease (MRD) in acute myeloid leukaemia (AML). Best Pract Res Clin Haematol. 2002. 15:105–118.
Article45. Langebrake C, Creutzig U, Dworzak M, et al. Residual disease monitoring in childhood acute myeloid leukemia by multiparameter flow cytometry: the MRD-AML-BFM Study Group. J Clin Oncol. 2006. 24:3686–3692.
Article46. Maurillo L, Buccisano F, Del Principe MI, et al. Toward optimization of postremission therapy for residual disease-positive patients with acute myeloid leukemia. J Clin Oncol. 2008. 26:4944–4951.
Article47. Lane S, Saal R, Mollee P, et al. A >or=1 log rise in RQ-PCR transcript levels defines molecular relapse in core binding factor acute myeloid leukemia and predicts subsequent morphologic relapse. Leuk Lymphoma. 2008. 49:517–523.
Article48. Corbacioglu A, Scholl C, Schlenk RF, et al. Prognostic impact of minimal residual disease in CBFB-MYH11-positive acute myeloid leukemia. J Clin Oncol. 2010. 28:3724–3729.
Article49. Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010. 11:543–552.
Article50. van der Velden VH, van der Sluijs-Geling A, Gibson BE, et al. Clinical significance of flowcytometric minimal residual disease detection in pediatric acute myeloid leukemia patients treated according to the DCOG ANLL97/MRC AML12 protocol. Leukemia. 2010. 24:1599–1606.
Article51. Krönke J, Schlenk RF, Jensen KO, et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011. 29:2709–2716.
Article52. Yin JA, O'Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood. 2012. 120:2826–2835.
Article53. Loken MR, Alonzo TA, Pardo L, et al. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: a report from Children's Oncology Group. Blood. 2012. 120:1581–1588.
Article54. Flohr T, Schrauder A, Cazzaniga G, et al. Minimal residual disease-directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the international multicenter trial AIEOP-BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia. 2008. 22:771–782.
Article55. Coustan-Smith E, Campana D. Immunologic minimal residual disease detection in acute lymphoblastic leukemia: a comparative approach to molecular testing. Best Pract Res Clin Haematol. 2010. 23:347–358.
Article56. Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009. 360:2730–2741.
Article57. Longacre TA, Foucar K, Crago S, et al. Hematogones: a multiparameter analysis of bone marrow precursor cells. Blood. 1989. 73:543–552.
Article58. Rimsza LM, Larson RS, Winter SS, et al. Benign hematogone-rich lymphoid proliferations can be distinguished from B-lineage acute lymphoblastic leukemia by integration of morphology, immunophenotype, adhesion molecule expression, and architectural features. Am J Clin Pathol. 2000. 114:66–75.
Article59. van Wering ER, van der Linden-Schrever BE, Szczepanski T, et al. Regenerating normal B-cell precursors during and after treatment of acute lymphoblastic leukaemia: implications for monitoring of minimal residual disease. Br J Haematol. 2000. 110:139–146.
Article60. McKenna RW, Washington LT, Aquino DB, Picker LJ, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) in 662 consecutive bone marrow specimens by 4-color flow cytometry. Blood. 2001. 98:2498–2507.
Article61. Luria D, Rosenthal E, Steinberg D, et al. Prospective comparison of two flow cytometry methodologies for monitoring minimal residual disease in a multicenter treatment protocol of childhood acute lymphoblastic leukemia. Cytometry B Clin Cytom. 2010. 78:365–371.
Article62. Neale GA, Coustan-Smith E, Stow P, et al. Comparative analysis of flow cytometry and polymerase chain reaction for the detection of minimal residual disease in childhood acute lymphoblastic leukemia. Leukemia. 2004. 18:934–938.
Article63. Kerst G, Kreyenberg H, Roth C, et al. Concurrent detection of minimal residual disease (MRD) in childhood acute lymphoblastic leukaemia by flow cytometry and real-time PCR. Br J Haematol. 2005. 128:774–782.
Article64. Malec M, Björklund E, Söderhäll S, et al. Flow cytometry and allele-specific oligonucleotide PCR are equally effective in detection of minimal residual disease in ALL. Leukemia. 2001. 15:716–727.
Article65. Irving J, Jesson J, Virgo P, et al. Establishment and validation of a standard protocol for the detection of minimal residual disease in B lineage childhood acute lymphoblastic leukemia by flow cytometry in a multi-center setting. Haematologica. 2009. 94:870–874.
Article66. Denys B, van der Sluijs-Gelling AJ, Homburg C, et al. Improved flow cytometric detection of minimal residual disease in childhood acute lymphoblastic leukemia. Leukemia. 2012. [Epub ahead of print].
Article67. Metzler M, Mann G, Monschein U, et al. Minimal residual disease analysis in children with t(12;21)-positive acute lymphoblastic leukemia: comparison of Ig/TCR rearrangements and the genomic fusion gene. Haematologica. 2006. 91:683–686.68. Zaliova M, Fronkova E, Krejcikova K, et al. Quantification of fusion transcript reveals a subgroup with distinct biological properties and predicts relapse in BCR/ABL-positive ALL: implications for residual disease monitoring. Leukemia. 2009. 23:944–951.
Article69. Boeckx N, Willemse MJ, Szczepanski T, et al. Fusion gene transcripts and Ig/TCR gene rearrangements are complementary but infrequent targets for PCR-based detection of minimal residual disease in acute myeloid leukemia. Leukemia. 2002. 16:368–375.
Article70. Brown P, McIntyre E, Rau R, et al. The incidence and clinical significance of nucleophosmin mutations in childhood AML. Blood. 2007. 110:979–985.
Article71. Meshinchi S, Woods WG, Stirewalt DL, et al. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood. 2001. 97:89–94.
Article72. Campana D, Coustan-Smith E. Detection of minimal residual disease in acute leukemia by flow cytometry. Cytometry. 1999. 38:139–152.
Article73. Inaba H, Coustan-Smith E, Cao X, et al. Comparative analysis of different approaches to measure treatment response in acute myeloid leukemia. J Clin Oncol. 2012. 30:3625–3632.
Article74. Yeoh AE, Ariffin H, Chai EL, et al. Minimal residual disease-guided treatment deintensification for children with acute lymphoblastic leukemia: results from the Malaysia-Singapore acute lymphoblastic leukemia 2003 study. J Clin Oncol. 2012. 30:2384–2392.
Article75. Panzer-Grümayer ER, Schneider M, Panzer S, Fasching K, Gadner H. Rapid molecular response during early induction chemotherapy predicts a good outcome in childhood acute lymphoblastic leukemia. Blood. 2000. 95:790–794.
Article76. Koh KN, Park M, Kim BE, et al. Prognostic significance of minimal residual disease detected by a simplified flow cytometric assay during remission induction chemotherapy in children with acute lymphoblastic leukemia. Korean J Pediatr. 2010. 53:957–964.
Article77. van der Velden VH, Jacobs DC, Wijkhuijs AJ, et al. Minimal residual disease levels in bone marrow and peripheral blood are comparable in children with T cell acute lymphoblastic leukemia (ALL), but not in precursor-B-ALL. Leukemia. 2002. 16:1432–1436.
Article78. Leung W, Pui CH, Coustan-Smith E, et al. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood. 2012. 120:468–472.
Article79. Lankester AC, Bierings MB, van Wering ER, et al. Preemptive alloimmune intervention in high-risk pediatric acute lymphoblastic leukemia patients guided by minimal residual disease level before stem cell transplantation. Leukemia. 2010. 24:1462–1469.
Article80. Coustan-Smith E, Song G, Clark C, et al. New markers for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2011. 117:6267–6276.
Article81. Bendall SC, Simonds EF, Qiu P, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011. 332:687–696.
Article82. Pedreira CE, Costa ES, Almeida J, et al. A probabilistic approach for the evaluation of minimal residual disease by multiparameter flow cytometry in leukemic B-cell chronic lymphoproliferative disorders. Cytometry A. 2008. 73A:1141–1150.
Article83. Fišer K, Sieger T, Schumich A, et al. Detection and monitoring of normal and leukemic cell populations with hierarchical clustering of flow cytometry data. Cytometry A. 2012. 81:25–34.
Article84. Liu X, Hsieh HB, Campana D, Bruce RH. A new method for high speed, sensitive detection of minimal residual disease. Cytometry A. 2012. 81:169–175.
Article85. van der Velden VH, Hochhaus A, Cazzaniga G, Szczepanski T, Gabert J, van Dongen JJ. Detection of minimal residual disease in hematologic malignancies by real-time quantitative PCR: principles, approaches, and laboratory aspects. Leukemia. 2003. 17:1013–1034.
Article86. Boyd SD, Marshall EL, Merker JD, et al. Measurement and clinical monitoring of human lymphocyte clonality by massively parallel VDJ pyrosequencing. Sci Transl Med. 2009. 1:12ra23.
Article87. Wu D, Sherwood A, Fromm JR, et al. High-throughput sequencing detects minimal residual disease in acute T lymphoblastic leukemia. Sci Transl Med. 2012. 4:134ra63.
Article88. Faham M, Zheng J, Moorhead M, et al. Deep sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012. [Epub ahead of print].
Article89. Coustan-Smith E, Ribeiro RC, Stow P, et al. A simplified flow cytometric assay identifies children with acute lymphoblastic leukemia who have a superior clinical outcome. Blood. 2006. 108:97–102.
Article90. Koh KN, Park M, Kim BE, et al. Prognostic significance of minimal residual disease detected by a simplified flow cytometric assay during remission induction chemotherapy in children with acute lymphoblastic leukemia. Korean J Pediatr. 2010. 53:957–964.
Article91. Schrappe M, Valsecchi MG, Bartram CR, et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood. 2011. 118:2077–2084.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Meeting Report: 2009 Symposium on Childhood Acute Lymphoblastic Leukemia - Update on the Diagnosis and Treatment for Acute Lymphoblastic Leukemia in Childhood & Adolescence; Seoul; Korea; June 27, 2009
- Advances in the Treatment of Childhood Acute Lymphoblastic Leukemia
- Recent advances in the treatment of pediatric acute leukemia
- Treatments for children and adolescents with AML
- Treatment for Childhood Acute Myelogenous Leukemia in Japan