J Korean Med Sci.
2010 Nov;25(11):1638-1645. 10.3346/jkms.2010.25.11.1638.
Early Motor Balance and Coordination Training Increased Synaptophysin in Subcortical Regions of the Ischemic Rat Brain
- Affiliations
-
- 1Department of Rehabilitation Medicine, Seoul National University Hospital, Seoul, Korea.
- 2Department of Rehabilitation Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. kysmart@amc.seoul.kr
- 3Department of Rehabilitation Medicine, Seoul National University Boramae Hospital, Seoul, Korea.
- 4Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1779235
- DOI: http://doi.org/10.3346/jkms.2010.25.11.1638
Abstract
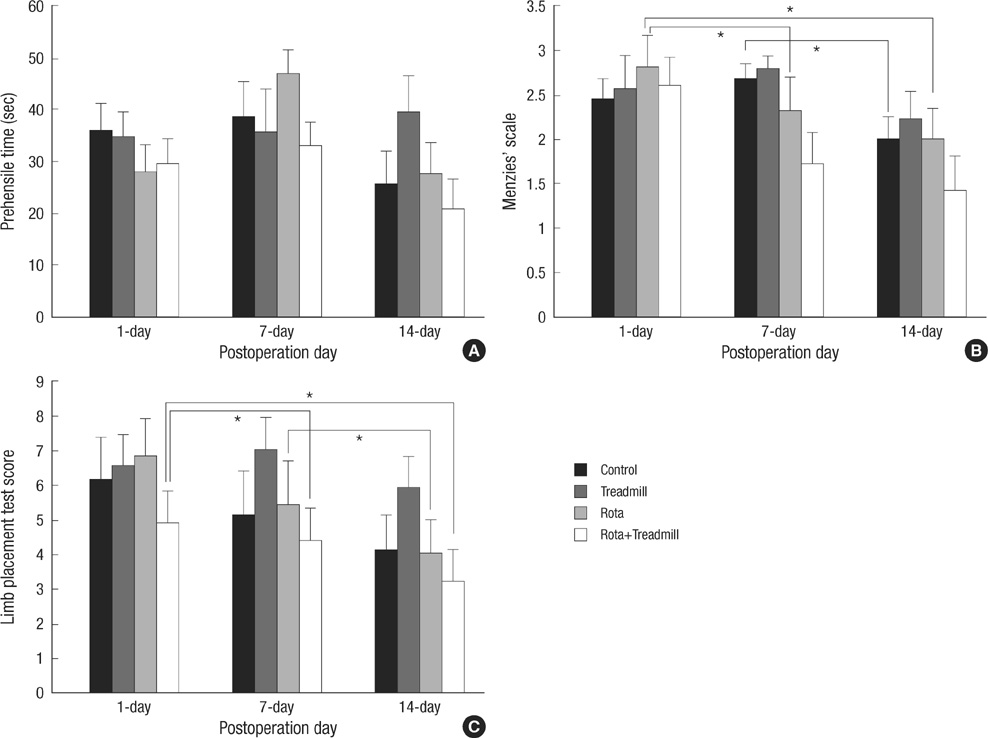
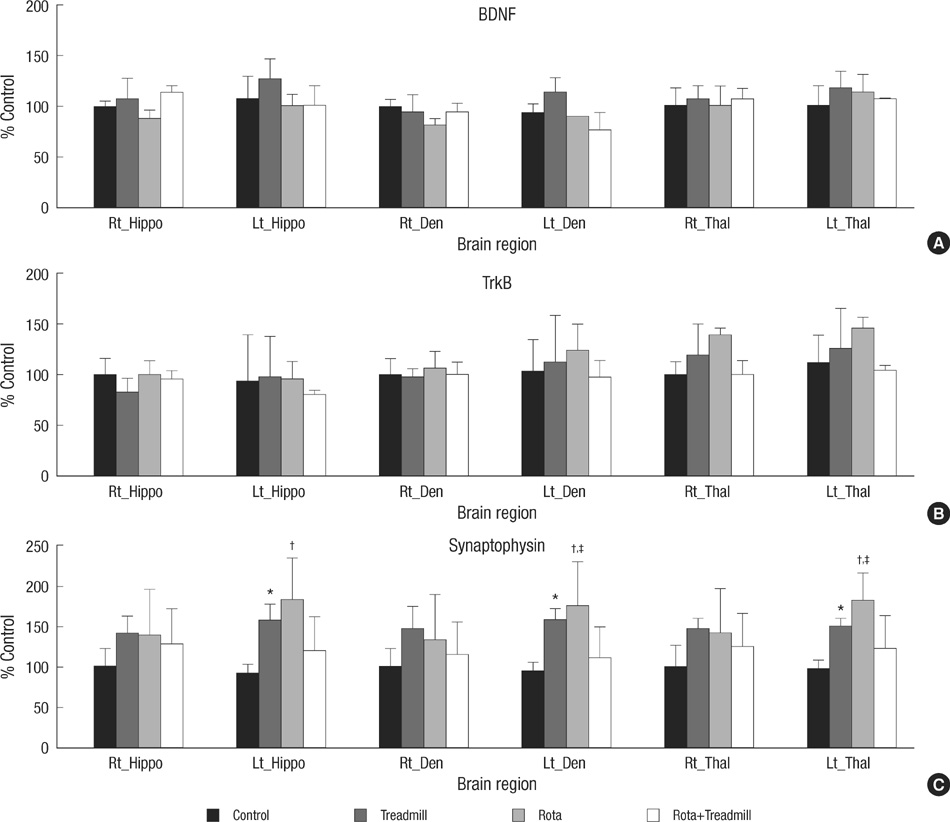
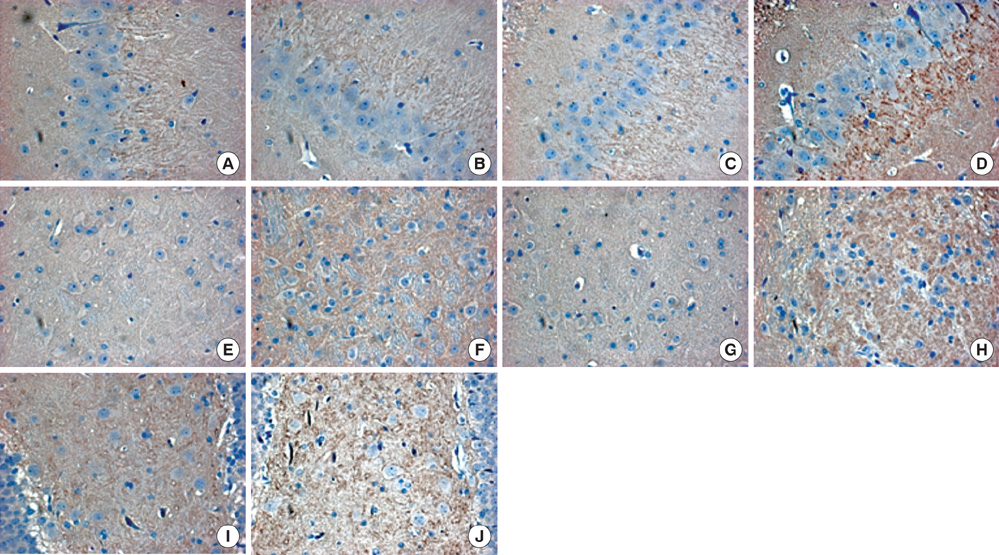
- The aim of this study was to evaluate the effect of early motor balance and coordination training on functional recovery and brain plasticity in an ischemic rat stroke model, compared with simple locomotor exercise. Adult male Sprague-Dawley rats with cortical infarcts were trained under one of four conditions: nontrained control, treadmill training, motor training on the Rota-rod, or both Rota-rod and treadmill training. All types of training were performed from post-operation day 1 to 14. Neurological and behavioral performance was evaluated by Menzies' scale, the prehensile test, and the limb placement test, at post-operation day 1, 7, and 14. Both Rota-rod and treadmill training increased the expression of synaptophysin in subcortical regions of the ischemic hemisphere including the hippocampus, dentate gyrus, and thalamus, but did not affect levels of brain-derived neurotrophic factor or tyrosin kinase receptor B. The Rota-rod training also improved Menzies' scale and limb placement test scores, whereas the simple treadmill training did neither. The control group showed significant change only in Menzies' scale score. This study suggests that early motor balance and coordination training may induce plastic changes in subcortical regions of the ischemic hemisphere after stroke accompanied with the recovery of sensorimotor performance.
Keyword
MeSH Terms
-
Animals
Brain Ischemia/metabolism/physiopathology
Brain-Derived Neurotrophic Factor/metabolism
Dentate Gyrus/metabolism
Disease Models, Animal
Hippocampus/metabolism
Immunohistochemistry
Male
Motor Activity
Neuronal Plasticity/physiology
Physical Conditioning, Animal
Physical Therapy Modalities
Rats
Rats, Sprague-Dawley
Receptor, trkB/metabolism
Stroke/*metabolism/physiopathology
Synaptophysin/*metabolism
Thalamus/metabolism
Time Factors
Figure
Cited by 1 articles
-
Low-Frequency Repetitive Transcranial Magnetic Stimulation in the Early Subacute Phase of Stroke Enhances Angiogenic Mechanisms in Rats
Yookyung Lee, Byung-Mo Oh, Sung-Hye Park, Tai Ryoon Han
Ann Rehabil Med. 2022;46(5):228-236. doi: 10.5535/arm.22040.
Reference
-
1. Johansson BB, Grabowski M. Functional recovery after brain infarction: plasticity and neural transplantation. Brain Pathol. 1994. 4:85–95.
Article2. Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995. 26:2135–2144.
Article3. Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996. 19:289–317.
Article4. Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000. 14:2919–2937.
Article5. Lindvall O, Kokaia Z, Bengzon J, Elmer E, Kokaia M. Neurotrophins and brain insults. Trends Neurosci. 1994. 17:490–496.
Article6. Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996. 16:4529–4535.
Article7. Ding Y, Li J, Clark J, Diaz FG, Rafols JA. Synaptic plasticity in thalamic nuclei enhanced by motor skill training in rat with transient middle cerebral artery occlusion. Neurol Res. 2003. 25:189–194.
Article8. Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996. 726:49–56.
Article9. van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999. 96:13427–13431.
Article10. Vaynman S, Ying Z, Gomez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J Neurosci Res. 2004. 76:356–362.
Article11. Ploughman M, Granter-Button S, Chernenko G, Tucker BA, Mearow KM, Corbett D. Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia. Neuroscience. 2005. 136:991–1001.
Article12. Kim MW, Bang MS, Han TR, Ko YJ, Yoon BW, Kim JH, Kang LM, Lee KM, Kim MH. Exercise increased BDNF and trkB in the contralateral hemisphere of the ischemic rat brain. Brain Res. 2005. 1052:16–21.
Article13. Klintsova AY, Dickson E, Yoshida R, Greenough WT. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 2004. 1028:92–104.
Article14. Griesbach GS, Gomez-Pinilla F, Hovda DA. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res. 2004. 1016:154–162.
Article15. Ding Y, Li J, Lai Q, Rafols JA, Luan X, Clark J, Diaz FG. Motor balance and coordination training enhances functional outcome in rat with transient middle cerebral artery occlusion. Neuroscience. 2004. 123:667–674.
Article16. Maldonado MA, Allred RP, Felthauser EL, Jones TA. Motor skill training, but not voluntary exercise, improves skilled reaching after unilateral ischemic lesions of the sensorimotor cortex in rats. Neurorehabil Neural Repair. 2008. 22:250–261.
Article17. Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997. 28:2060–2065. discussion 6.
Article18. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989. 20:84–91.
Article19. Combs DJ, D'Alecy LG. Motor performance in rats exposed to severe forebrain ischemia: effect of fasting and 1,3-butanediol. Stroke. 1987. 18:503–511.
Article20. Menzies SA, Hoff JT, Betz AL. Middle cerebral artery occlusion in rats: a neurological and pathological evaluation of a reproducible model. Neurosurgery. 1992. 31:100–106.21. Jeong SW, Chu K, Jung KH, Kim SU, Kim M, Roh JK. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003. 34:2258–2263.
Article22. Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve. 2001. 24:1000–1019.
Article23. Jones EG, Pons TP. Thalamic and brainstem contributions to large-scale plasticity of primate somatosensory cortex. Science. 1998. 282:1121–1125.
Article24. Zhao LR, Risedal A, Wojcik A, Hejzlar J, Johansson BB, Kokaia Z. Enriched environment influences brain-derived neurotrophic factor levels in rat forebrain after focal stroke. Neurosci Lett. 2001. 305:169–172.
Article25. Kokaia Z, Zhao Q, Kokaia M, Elmer E, Metsis M, Smith ML, Siesjo BK, Lindvall O. Regulation of brain-derived neurotrophic factor gene expression after transient middle cerebral artery occlusion with and without brain damage. Exp Neurol. 1995. 136:73–88.
Article26. Zhao LR, Mattsson B, Johansson BB. Environmental influence on brain-derived neurotrophic factor messenger RNA expression after middle cerebral artery occlusion in spontaneously hypertensive rats. Neuroscience. 2000. 97:177–184.
Article27. Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006. 1070:124–130.
Article28. Yang YR, Wang RY, Wang PS. Early and late treadmill training after focal brain ischemia in rats. Neurosci Lett. 2003. 339:91–94.
Article29. Risedal A, Zeng J, Johansson BB. Early training may exacerbate brain damage after focal brain ischemia in the rat. J Cereb Blood Flow Metab. 1999. 19:997–1003.
Article30. Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004. 125:129–139.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Balance and Coordination Training for Brain Disorders
- Neuroanatomical Basis of Apraxia
- Neuroplasticity Induced by Robot-assisted Gait Training in a Stroke Patient: A case report
- Differential Expression Levels of Synaptophysin through Developmental Stages in Cerebral Cortices of Mouse Brain
- White matter plasticity in the cerebellum of elite basketball athletes