J Korean Med Sci.
2005 Jun;20(3):438-444. 10.3346/jkms.2005.20.3.438.
The Effect of Simvastatin on the Proliferation and Differentiation of Human Bone Marrow Stromal Cells
- Affiliations
-
- 1Department of Internal Medicine, The Catholic University of Korea, College of Medicine, Seoul, Korea. mikang@catholic.ac.kr
- 2Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Pyungchon Sacred Heart Hospital, Hallym University, College of Medicine, Anyang, Korea.
- KMID: 1778503
- DOI: http://doi.org/10.3346/jkms.2005.20.3.438
Abstract
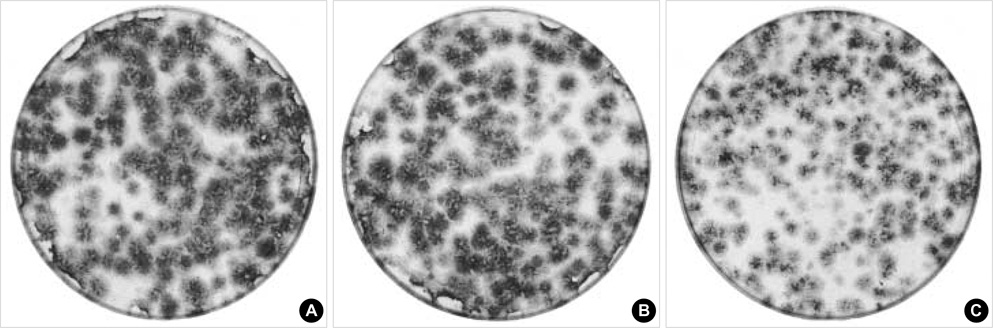
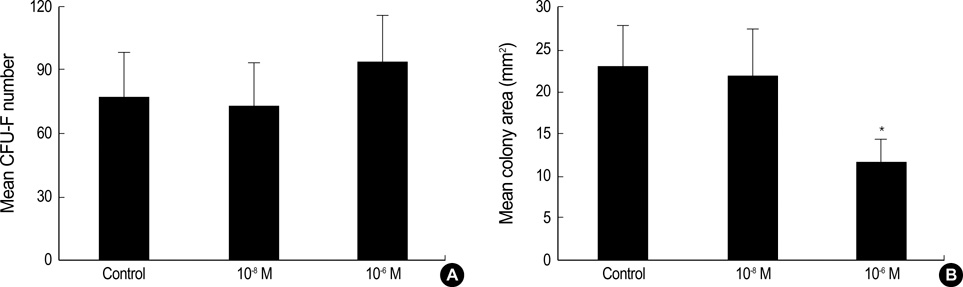
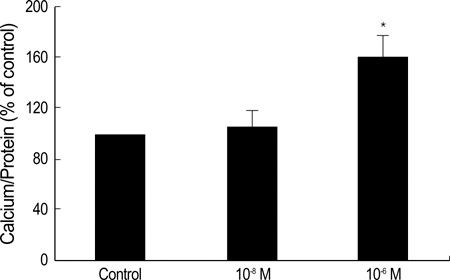
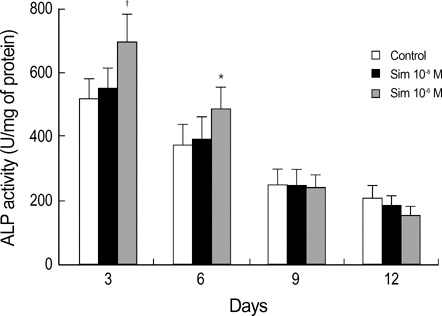
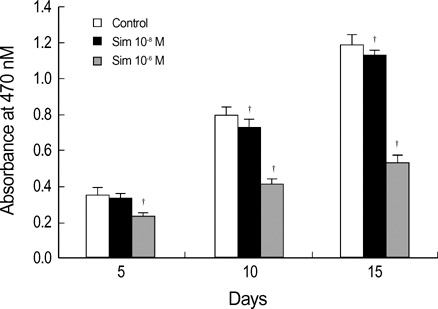
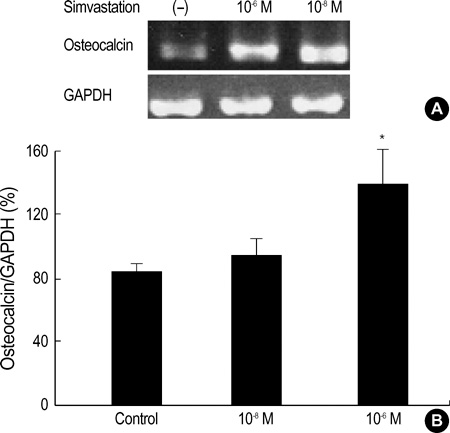
- Statins have been postulated to affect the bone metabolism. Recent experimental and epidemiologic studies have suggested that statins may also have bone protective effects. This study assessed the effects of simvastatin on the proliferation and differentiation of human bone marrow stromal cells (BMSCs) in an ex vivo culture. The bone marrow was obtained from healthy donors. Mononuclear cells were isolated and cultured to osteoblastic lineage. In the primary culture, 10(-6) M simvastatin diminished the mean size of the colony forming units-fibroblastic (CFU-Fs) and enhanced matrix calcification. At near confluence, the cells were sub-cultured. Thereafter, the alkaline phosphatase (ALP) activities of each group were measured by the time course of the secondary culture. Simvastatin increased the ALP activity in a dose dependent manner, and this stimulatory effect was more evident during the early period of culture. A 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT) assay was performed during the secondary culture in order to estimate the effect of simvastatin on the proliferation of human BMSCs. When compared to the control group, simvastatin significantly decreased the proliferation of cells of each culture well. 10(-6) M of simvastatin also significantly enhanced the osteocalcin mRNA expression level. This study shows that simvastatin has a stimulatory effect on bone formation through osteoblastic differentiation, and has an inhibitory effect on the proliferative potential of human BMSCs.
Keyword
MeSH Terms
-
Alkaline Phosphatase/metabolism
Bone Marrow Cells/cytology/*drug effects/metabolism
Calcification, Physiologic/drug effects
Cell Differentiation/*drug effects
Cell Proliferation/*drug effects
Cells, Cultured
Colony-Forming Units Assay
Comparative Study
Dose-Response Relationship, Drug
Gene Expression/drug effects
Humans
Hydroxymethylglutaryl-CoA Reductase Inhibitors/pharmacology
RNA, Messenger/genetics/metabolism
Research Support, Non-U.S. Gov't
Reverse Transcriptase Polymerase Chain Reaction
Simvastatin/*pharmacology
Stromal Cells/cytology/drug effects/metabolism
Time Factors
Figure
Reference
-
1. Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999. 286:1946–1949.
Article2. Edwards CJ, Russell RG, Spector TD. Statins and bone: myth or reality? Calcif Tissue Int. 2001. 69:63–66.
Article3. Edwards CJ, Hart DJ, Spector TD. Oral statins and increased bone mineral density in postmenopausal women. Lancet. 2000. 355:2218–2219.4. Meier CR, Schlienger RG, Kraenzlin ME, Schlegel B, Jick H. HMG-CoA reductase inhibitors and the risk of fractures. JAMA. 2000. 283:3205–3210.
Article5. Chan KA, Andrade SE, Boles M, Buist DS, Chase GA, Donahue JG, Goodman MJ, Gurwitz JH, LaCroix AZ, Platt R. Inhibitors of hydroxymethylglutaryl-coenzyme A reductase and risk of fracture among older women. Lancet. 2000. 355:2185–2188.
Article6. Wang PS, Solomon DH, Mogun H, Avorn J. HMG-CoA reductase inhibitors and the risk of hip fractures in elderly patients. JAMA. 2000. 283:3211–3216.
Article7. Van Staa TP, Wegman S, De Vries F, Leufkens B, Cooper C. Use of statins and risk of fractures. JAMA. 2001. 285:1850–1855.
Article8. Bjarnason NH, Riis BJ, Christiansen C. The effect of fluvastatin on parameters of bone remodeling. Osteoporos Int. 2001. 12:380–384.
Article9. Sirola J, Honkanen R, Kröger H, Jurvelin JS, Mäenpää P, Saarikoski S. Relation of statin use and bone loss: A prospective population-based cohort study in early postmenopausal women. Osteoporos Int. 2002. 13:537–541.
Article10. Rejnmark L, Buus NH, Vestergaard P, Heickendorff L, Andreasen F, Larsen ML, Mosekilde L. Effects of simvastatin on bone turnover and BMD: a 1-year randomized controlled trial in postmenopausal osteopenic women. J Bone Miner Res. 2004. 19:737–744.
Article11. Oxlund H, Dalstra M, Andreassen T. Statin given perorally to adult rats increases cancellous bone mass and compressive strength. Calcif Tissue Int. 2001. 69:299–304.
Article12. Maritz FJ, Conradie MM, Hulley PA, Gopal R, Hough S. Effect of statins on bone mineral density and bone histomorphometry in rodents. Arterioscler Thromb Vasc Biol. 2001. 21:1636–1641.
Article13. Skoglund B, Forslund C, Aspenberg P. Simvastatin improves fracture healing in mice. J Bone Miner Res. 2002. 17:2004–2008.
Article14. Maeda T, Matsunuma A, Kawane T, Horiuchi N. Simvastatin promotes osteoblast differentiation and mineralization in MC3T3-E1 cells. Biochem Biophys Res Commun. 2001. 280:874–877.
Article15. Sugiyama M, Kodama T, Konishi K, Abe K, Asami S, Oikawa S. Compactin and simvastatin, but not pravastatin induce bone morphogenetic protein-2 in human osteosarcoma cells. Biochem Biophys Res Commun. 2000. 271:688–692.
Article16. Philips BW, Belmonte N, Vernochet C, Ailhaud G, Dani C. Compactin enhances osteogenesis in murine embryonic stems cells. Biochem Biophys Res Commun. 2001. 284:478–484.17. Milne M, Kang MI, Quail JM, Baran DT. Thyroid hormone excess increases insulin-like growth factor I transcripts in bone marrow cell cultures: divergent effects on vertebral and femoral cell cultures. Endocrinology. 1998. 139:2527–2534.
Article18. Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985. 150:76–85.
Article19. Puzas JE, Brand JS. Bone cell phosphotyrosine phosphatase: Characterization and regulation by calcitropic hormones. Endocrinology. 1985. 116:2463–2468.
Article20. Mossmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983. 65:55–63.21. Ohnaka K, Shimoda S, Nawata H, Shimokawa H, Kaibuchi K, Iwamoto Y, Takayanagi R. Pitavastatin enhanced BMP-2 and osteocalcin expression by inhibition of Rho-associated kinase in human osteoblasts. Biochem Biophys Res Commun. 2001. 287:337–342.
Article22. Mundy GR. Statins and their potential for osteoporosis. Bone. 2001. 29:495–497.
Article23. Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atrovastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol. 2004. 94:1140–1146.24. Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987. 20:263–272.
Article25. Friedenstein AJ, Chailakhyan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970. 3:393–403.
Article26. Erben RG, Scutt AM, Miao D, Kollenkirchen U, Haberey M. Short-term treatment of rats with high-dose 1,25-dihydroxyvitamin D3 stimulates bone formation and increases the number of osteoblast precursor cells in bone marrow. Endocrinology. 1997. 138:4629–4635.27. Weinreb M, Suponitzky I, Keila S. Systemic administration of an anabolic dose of PGE2 in young rats increases the osteogenic capacity of bone marrow. Bone. 1997. 20:521–526.
Article28. Lecanda F, Avioli LV, Cheng SL. Regulation of bone matrix protein expression and induction of differentiation of human osteoblasts and human bone marrow stromal cells by bone morphogenetic protein-2. J Cell Biochem. 1997. 265:386–396.
Article29. Chaudhari A, Ron E, Rethman MP. Recombinant human bone morphogenetic protein-2 stimulates differentiation in primary cultures of fetal rat calvarial osteoblasts. Mol Cell Biochem. 1997. 167:31–39.30. Fromigue O, Marie PJ, Lomri A. Bone morphogenetic protein-2 and transforming growth factor-beta2 interact to modulate human bone marrow stromal cell proliferation and differentiation. J Cell Biochem. 1998. 68:411–426.31. Hock JM, Onyia J, Bidwell J. Comparisons of in vivo and in vitro models of the response of osteoblasts to hormonal regulation with aging. Calcif Tissue Int. 1995. 56:Suppl 1. S44–S47.
Article32. Nishida S, Yamaguchi A, Tanizawa T, Endo N, Mashiba T, Uchiyama Y, Suda T, Yoshiki S, Takahashi HE. Increased bone formation by intermittent parathyroid hormone administration is due to the stimulation of proliferation and differentiation of osteoprogenitor cells in the bone marrow. Bone. 1994. 15:717–723.33. Nakajima A, Shimoji N, Shiomi K, Shimizu S, Moriya H, Einhorn TA, Yamazaki M. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1-34). J Bone Miner Res. 2002. 17:2038–2047.
Article34. Canalis E. Insulin like growth factors and the local regulation of bone formation. Bone. 1993. 14:273–276.
Article35. Schmid C, Steiner T, Froesch ER. Insulin-like growth factor I supports differentiation of cultured osteoblast-like cells. FEBS Lett. 1984. 173:48–52.
Article36. Chung YS, Lee MD, Lee SK, Kim HM, Fitzpatrick LA. HMG-CoA reductase inhibitors increase BMD in type 2 diabetes mellitus patients. J Clin Endocrinol Metab. 2000. 85:1137–1142.
Article37. Pasco JA, Kotowicz MA, Henry MJ, Sanders KM, Nicholson GC. Statin use, bone mineral density, and fracture risk: Geelong Osteoporosis Study. Arch Intern Med. 2002. 162:537–540.38. Reid IR, Hague W, Emberson J, Baker J, Tonkin A, Hunt D, MacMahon S, Sharpe N. Effect of pravastatin on frequency of fracture in the LIPID study: secondary analysis of a randomised controlled trial. Lancet. 2001. 357:509–512.39. Mostaza JM, De la Piedra C, Curiel MD, Pena R, Lahoz C. Pravastatin therapy increases procollagen I N-terminal propeptide (PINP), a marker of bone formation in post-menopausal women. Clin Chim Acta. 2001. 308:133–137.
Article40. Chan MH, Mak TW, Chiu RW, Chow CC, Chan IH, Lam CW. Simvastatin increases serum osteocalcin concentration in patients treated for hypercholesterolaemia. J Clin Endocrinol Metab. 2001. 86:4556–4559.41. Stein EA, Farnier M, Waldstreicher J, Mercuri M. Effects of statins on biomarkers of bone metabolism: a randomised trial. Nutr Metab Cardiovasc Dis. 2001. 11:84–87.42. Rejnmark L, Buus NH, Vestergaard P, Andreasen F, Larsen ML, Mosekilde L. Statins decrease bone turnover in postmenopausal women: a cross-sectional study. Eur J Clin Invest. 2002. 32:581–589.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Human Bone Marrow Stromal Cells with Fibroblasts in Cell Proliferation and Collagen Synthesis
- Effects of simvastatin on the proliferation and apoptosis of human endometrial stromal cells from women with endometriosis
- Clonal Heterogeneity of Human Bone Marrow-Derived Stromal Cells
- Effect of Dexamethasone and 1,25(OH)2D3 on Proliferation and Osteogenic Differentiation of Cultured Human Bone Marrow Stromal Cells
- Effects of dexamethasone on proliferation, collagen synthesis and alkaline phosphatase activity of normal human bone marrow stromal cells