J Korean Med Sci.
2010 Mar;25(3):405-417. 10.3346/jkms.2010.25.3.405.
DNA Methylation Patterns of Ulcer-Healing Genes Associated with the Normal Gastric Mucosa of Gastric Cancers
- Affiliations
-
- 1Department of Microbiology, College of Medicine, The Catholic University of Korea, Seoul, Korea. rhyumung@catholic.ac.kr
- 2Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Clinical Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 1778036
- DOI: http://doi.org/10.3346/jkms.2010.25.3.405
Abstract
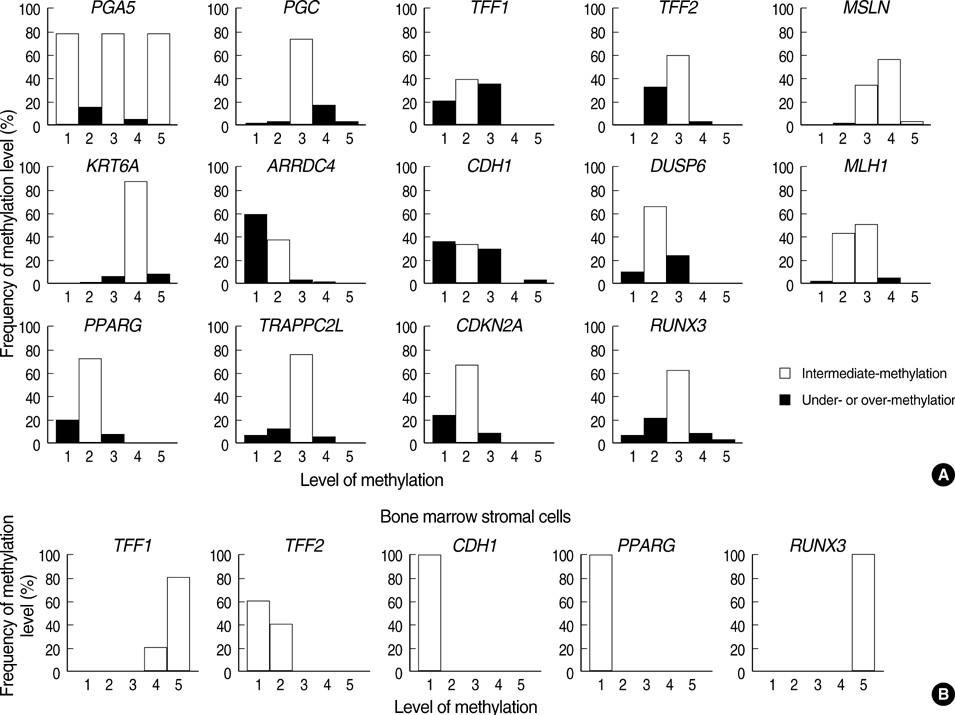
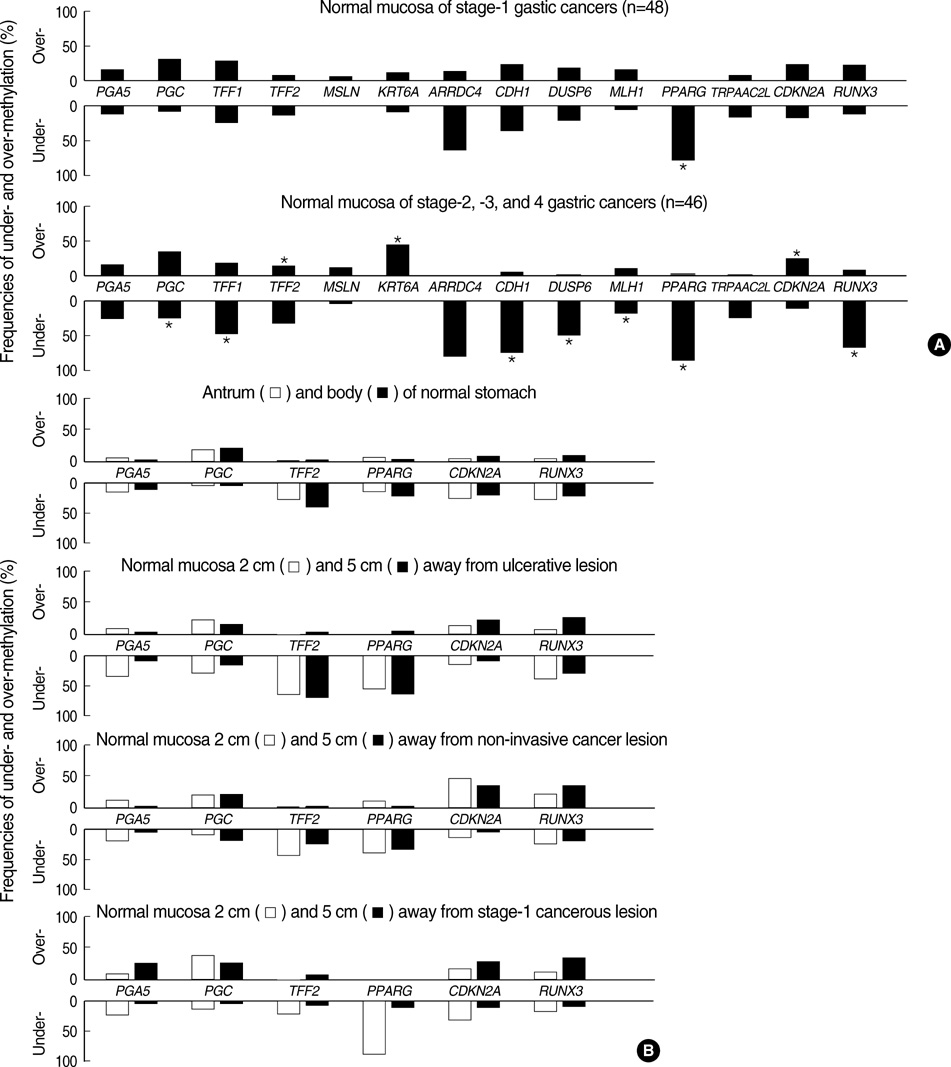
- Recent evidence suggests that gastric mucosal injury induces adaptive changes in DNA methylation. In this study, the methylation status of the key tissue-specific genes in normal gastric mucosa of healthy individuals and cancer patients was evaluated. The methylation-variable sites of 14 genes, including ulcer-healing genes (TFF1, TFF2, CDH1, and PPARG), were chosen from the CpG-island margins or non-island CpGs near the transcription start sites. The healthy individuals as well as the normal gastric mucosa of 23 ulcer, 21 non-invasive cancer, and 53 cancer patients were examined by semiquantitative methylation-specific polymerase chain reaction (PCR) analysis. The ulcer-healing genes were concurrently methylated with other genes depending on the presence or absence of CpG-islands in the normal mucosa of healthy individuals. Both the TFF2 and PPARG genes were frequently undermethylated in ulcer patients. The over- or intermediate-methylated TFF2 and undermethylated PPARG genes was more common in stage-1 cancer patients (71%) than in healthy individuals (10%; odds ratio [OR], 21.9) and non-invasive cancer patients (21%; OR, 8.9). The TFF2-PPARG methylation pattern of cancer patients was stronger in the older-age group (> or =55 yr; OR, 43.6). These results suggest that the combined methylation pattern of ulcer-healing genes serves as a sensitive marker for predicting cancer-prone gastric mucosa.
Keyword
MeSH Terms
-
Biological Markers/metabolism
Cadherins/genetics
CpG Islands
*DNA Methylation
Female
*Gastric Mucosa/pathology/physiology
Gene Expression Regulation, Neoplastic
Growth Substances/genetics
Humans
Male
Middle Aged
Neoplasm Invasiveness
PPAR gamma/genetics
Peptides/genetics
*Stomach Neoplasms/genetics/pathology
*Stomach Ulcer/genetics/pathology
Tumor Suppressor Proteins/genetics
Wound Healing/*genetics
Biological Markers
Cadherins
Growth Substances
PPAR gamma
Peptides
Tumor Suppressor Proteins
Figure
Reference
-
1. Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002. 16:6–21.
Article2. Eckhardt F, Lewin J, Cortese R, Rakyan VK, Attwood J, Burger M, Burton J, Cox TV, Davies R, Down TA, Haefliger C, Horton R, Howe K, Jackson DK, Kunde J, Koenig C, Liddle J, Niblett D, Otto T, Pettett R, Seemann S, Thompson C, West T, Rogers J, Olek A, Berlin K, Beck S. DNA methylation profiling of human chromosomes 6, 20 and 22. Nat Genet. 2006. 38:1378–1385.
Article3. Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007. 133:659–672.
Article4. Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004. 306:1568–1571.
Article5. Yatabe Y, Tavare S, Shibata D. Investigating stem cells in human colon by using methylation patterns. Proc Natl Acad Sci USA. 2001. 98:10839–10844.
Article6. Turker MS. Gene silencing in mammalian cells and the spread of DNA methylation. Oncogene. 2002. 21:5388–5393.
Article7. Kang MI, Rhyu MG, Kim YH, Jung YC, Hong SJ, Cho CS, Kim HS. The length of CpG islands is associated with the distribution of Alu and L1 retroelements. Genomics. 2006. 87:580–590.
Article8. Misteli T. Beyond the sequence: cellular organization of genome function. Cell. 2007. 128:787–800.
Article9. Leung WK, Yu J, Chan FK, To KF, Chan MW, Ebert MP, Ng EK, Chung SC, Malfertheiner P, Sung JJ. Expression of trefoil peptides (TFF1, TFF2, and TFF3) in gastric carcinomas, intestinal metaplasia, and non-neoplastic gastric tissues. J Pathol. 2002. 197:582–588.
Article10. Demetter P, De Vos M, Van Damme N, Baeten D, Elewaut D, Vermeulen S, Mareel M, Bullock G, Mielants H, Verbruggen G, De Keyser F, Veys EM, Cuvelier CA. Focal up-regulation of E-cadherincatenin complex in inflamed bowel mucosa but reduced expression in ulcer-associated cell lineage. Am J Clin Pathol. 2000. 114:364–370.
Article11. Shimada T, Fujii Y, Koike T, Tabei K, Namatame T, Yamagata M, Tajima A, Yoneda M, Terano A, Hiraishi H. Peroxisome proliferator-activated receptor gamma (PPARgamma) regulates trefoil factor family 2 (TFF2) expression in gastric epithelial cells. Int J Biochem Cell Biol. 2007. 39:626–637.12. Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969. 3:87–97.
Article13. Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Flejou JF, Geboes K, Hattori T, Hirota T, Itabashi M, Iwafuchi M, Iwashita A, Kim YI, Kirchner T, Klimpfinger M, Koike M, Lauwers GY, Lewin KJ, Oberhuber G, Offner F, Price AB, Rubio CA, Shimizu M, Shimoda T, Sipponen P, Solcia E, Stolte M, Watanabe H, Yamabe H. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000. 47:251–255.14. Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M. AJCC cancer staging manual. 2002. 6th ed. Berlin Heidelberg New York: Springer Verlag.15. Broutet N, Plebani M, Sakarovitch C, Sipponen P, Megraud F. Pepsinogen A, pepsinogen C, and gastrin as markers of atrophic chronic gastritis in European dyspeptics. Br J Cancer. 2003. 88:1239–1247.
Article16. Kim YH, Hong SJ, Jung YC, Kim SJ, Seo EJ, Choi SW, Rhyu MG. The 5'-end transitional CpGs between the CpG islands and retroelements are hypomethylated in association with loss of heterozygosity in gastric cancers. BMC Cancer. 2006. 6:180–197.
Article17. Osaki M, Moriyama M, Adachi K, Nakada C, Takeda A, Inoue Y, Adachi H, Sato K, Oshimura M, Ito H. Expression of RUNX3 protein in human gastric mucosa, intestinal metaplasia and carcinoma. Eur J Clin Invest. 2004. 34:605–612.
Article18. Frierson HF Jr, Moskaluk CA, Powell SM, Zhang H, Cerilli LA, Stoler MH, Cathro H, Hampton GM. Large-scale molecular and tissue microarray analysis of mesothelin expression in common human carcinomas. Hum Pathol. 2003. 34:605–609.
Article19. Yamamoto Y, Sakamoto M, Fujii G, Kanetaka K, Asaka M, Hirohashi S. Cloning and characterization of a novel gene, DRH1, down-regulated in advanced human hepatocellular carcinoma. Clin Cancer Res. 2001. 7:297–303.20. Lang R, Hammer M, Mages J. DUSP meet immunology: dual specificity MAPK phosphatases in control of the inflammatory response. J Immunol. 2006. 177:7497–7504.
Article21. Camilo R, Capelozzi VL, Siqueira SA, Del Carlo BF. Expression of p63, keratin 5/6, keratin 7, and surfactant-A in non-small cell lung carcinomas. Hum Pathol. 2006. 37:542–546.
Article22. Zhang QH, Ye M, Wu XY, Ren SX, Zhao M, Zhao CJ, Fu G, Shen Y, Fan HY, Lu G, Zhong M, Xu XR, Han ZG, Zhang JW, Tao J, Huang QH, Zhou J, Hu GX, Gu J, Chen SJ, Chen Z. Cloning and functional analysis of cDNAs with open reading frames for 300 previously undefined genes expressed in CD34+ hematopoietic stem/progenitor cells. Genome Res. 2000. 10:1546–1560.
Article23. Kang MI, Kim HS, Jung YC, Kim YH, Hong SJ, Kim MK, Baek KH, Kim CC, Rhyu MG. Transitional CpG methylation between promoters and retroelements of tissue-specific genes during human mesenchymal cell differentiation. J Cell Biochem. 2007. 102:224–239.
Article24. Jung YC, Hong SJ, Kim YH, Kim SJ, Kang SJ, Choi SW, Rhyu MG. Chromosomal losses are associated with hypomethylation of the gene-control regions in the stomach with a low number of active genes. J Korean Med Sci. 2008. 23:1068–1089.
Article25. Hong SJ, Kim YH, Choi YD, Min KO, Choi SW, Rhyu MG. Relationship between the extent of chromosomal losses and the pattern of CpG methylation in gastric carcinomas. J Korean Med Sci. 2005. 20:790–805.
Article26. Hong SJ, Kang MI, Oh JH, Jung YC, Kim YH, Kim SJ, Choi SH, Seo EJ, Choi SW, Rhyu MG. DNA methylation and expression patterns of key tissue-specific genes in adult stem cells and stomach tissues. J Korean Med Sci. 2009. 24:918–929.
Article27. Noer A, Sorensen AL, Boquest AC, Collas P. Stable CpG hypomethylation of adipogenic promoters in freshly isolated, cultured, and differentiated mesenchymal stem cells from adipose tissue. Mol Biol Cell. 2006. 17:3543–3556.
Article28. Schlesinger Y, Straussman R, Keshet I, Farkash S, Hecht M, Zimmerman J, Eden E, Yakhini Z, Ben Shushan E, Reubinoff BE, Bergman Y, Simon I, Cedar H. Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nat Genet. 2007. 39:232–236.
Article29. Garcia SB, Park HS, Novelli M, Wright NA. Field cancerization, clonality, and epithelial stem cells: the spread of mutated clones in epithelial sheets. J Pathol. 1999. 187:61–81.
Article30. Maekita T, Nakazawa K, Mihara M, Nakajima T, Yanaoka K, Iguchi M, Arii K, Kaneda A, Tsukamoto T, Tatematsu M, Tamura G, Saito D, Sugimura T, Ichinose M, Ushijima T. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006. 12:989–995.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Periodic Methylation Patterns in the Background Mucosa of Gastric Cancer
- RUNX3 Methylation Status in Colonic Carcinoma and Adenoma
- CpG Island Hypermethylation in Gastric Carcinoma and Its Premalignant Lesions
- CpG Island Methylation According to the Histologic Patterns of Early Gastric Adenocarcinoma
- Topographic Difference of Inflammatory Reactions in Gastric Mucosa in Various Helicobacter pylori-Associated Diseases