J Korean Med Sci.
2014 Apr;29(4):512-518. 10.3346/jkms.2014.29.4.512.
The Effect of Preceding Biopsy on Complete Endoscopic Resection in Rectal Carcinoid Tumor
- Affiliations
-
- 1Department of Internal Medicine, Konkuk University School of Medicine, Seoul, Korea. inksung@kuh.ac.kr
- KMID: 1774456
- DOI: http://doi.org/10.3346/jkms.2014.29.4.512
Abstract
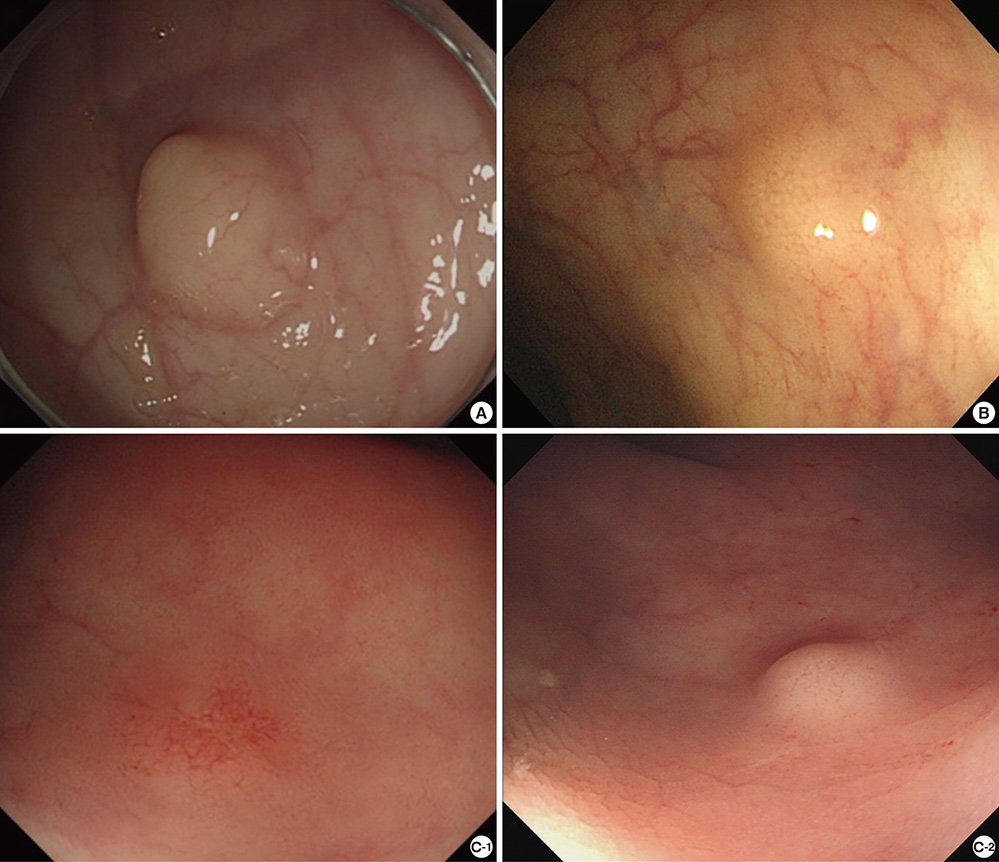
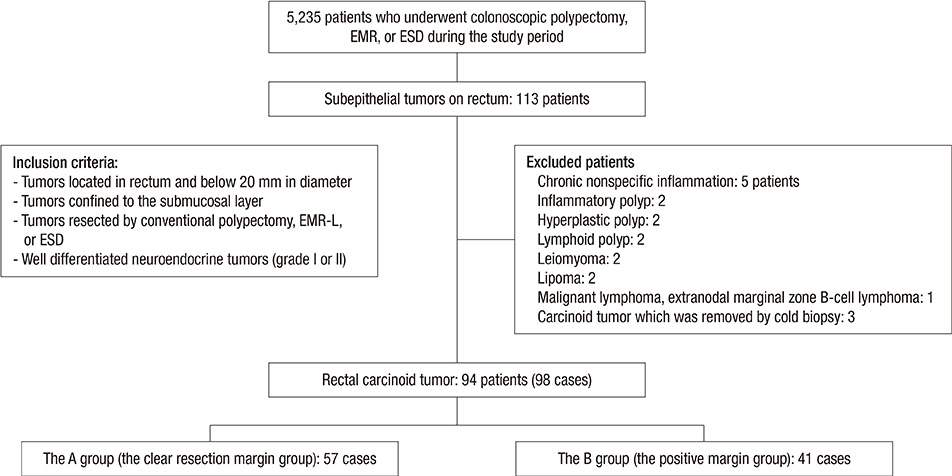
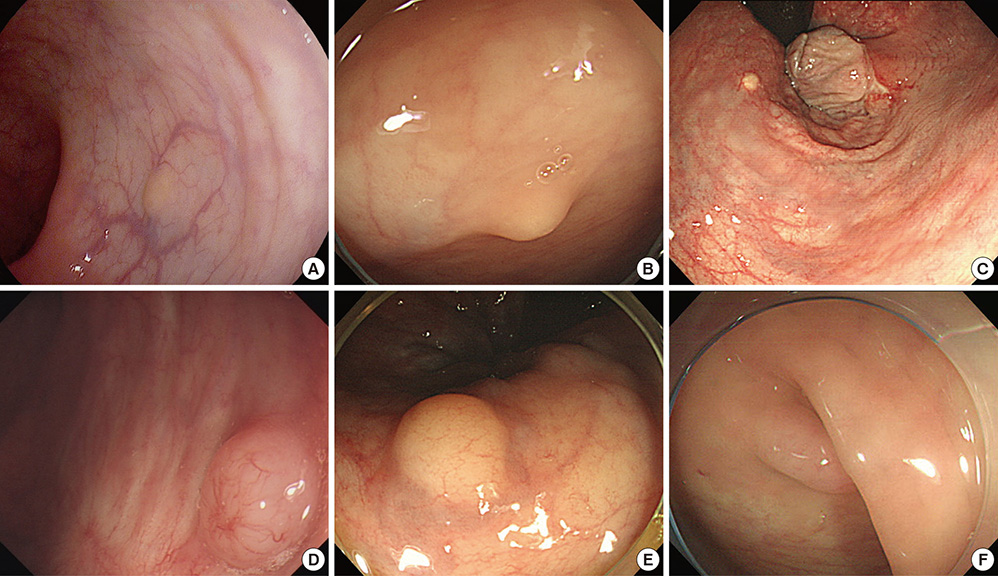
- Biopsy of rectal carcinoid tumor is commonly taken before endoscopic resection. However the preceding biopsy can inhibit complete resection by causing blurred tumor border and fibrosis of the tissue. The objective of the study was to investigate the effect of preceding biopsy on complete endoscopic resection in rectal carcinoid tumor. It was also determined if rectal carcinoid tumors can be macroscopically distinguished by endoscopy. We reviewed retrospectively the records of patients with rectal carcinoid tumor who had undergone an endoscopic treatment at our hospital, during a 7-yr period. The resection margin was clear in 57 of 98 cases. The preceding biopsy was taken in 57 cases and the biopsy was significantly associated with the risk of incomplete tumor resection (OR, 3.696; 95% CI, 1.528-8.938, P = 0.004). In 95.9% of the cases, it was possible to suspect a carcinoid tumor by macroscopic appearance during initial endoscopy. The preceding biopsy may disturb complete resection of rectal carcinoid tumor. In most cases, the carcinoid tumor could be suspected by macroscopic appearance. Therefore the preceding biopsy is not essential, and it may be avoided for the complete resection.
MeSH Terms
Figure
Reference
-
1. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003; 97:934–959.2. Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010; 39:707–712.3. Pinchot SN, Holen K, Sippel RS, Chen H. Carcinoid tumors. Oncologist. 2008; 13:1255–1269.4. Pascarella MR, McCloskey D, Jenab-Wolcott J, Vala M, Rovito M, McHugh J. Large cell neuroendocrine carcinoma of the colon: a rare and aggressive tumor. J Gastrointest Oncol. 2011; 2:250–253.5. Kang H, O'Connell JB, Leonardi MJ, Maggard MA, McGory ML, Ko CY. Rare tumors of the colon and rectum: a national review. Int J Colorectal Dis. 2007; 22:183–189.6. Shebani KO, Souba WW, Finkelstein DM, Stark PC, Elgadi KM, Tanabe KK, Ott MJ. Prognosis and survival in patients with gastrointestinal tract carcinoid tumors. Ann Surg. 1999; 229:815–821.7. Ramage JK, Goretzki PE, Manfredi R, Komminoth P, Ferone D, Hyrdel R, Kaltsas G, Kelestimur F, Kvols L, Scoazec JY, et al. Consensus guidelines for the management of patients with digestive neuroendocrine tumours: well-differentiated colon and rectum tumour/carcinoma. Neuroendocrinology. 2008; 87:31–39.8. Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci. 2004; 1014:13–27.9. Vilallonga R, Espín Basany E, López Cano M, Landolfi S, Armengol Carrasco M. Neuroendocrine carcinomas of the colon and rectum: a unit's experience over six years. Rev Esp Enferm Dig. 2008; 100:11–16.10. Cesar D, Zanatto RM, da Silva MV, Golçalves R, de Mello EL, de Jesus JP. Colon and rectum neuroendocrine tumors: experience of the National Cancer Institute in Brazil. Arq Bras Cir Dig. 2013; 26:36–39.11. Kim MS, Hur H, Min BS, Baik SH, Lee KY, Kim NK. Clinical outcomes for rectal carcinoid tumors according to a new (AJCC 7th edition) TNM staging system: a single institutional analysis of 122 patients. J Surg Oncol. 2013; 107:835–841.12. Onozato Y, Kakizaki S, Iizuka H, Sohara N, Mori M, Itoh H. Endoscopic treatment of rectal carcinoid tumors. Dis Colon Rectum. 2010; 53:169–176.13. Chagpar R, Chiang YJ, Xing Y, Cormier JN, Feig BW, Rashid A, Chang GJ, You YN. Neuroendocrine tumors of the colon and rectum: prognostic relevance and comparative performance of current staging systems. Ann Surg Oncol. 2013; 20:1170–1178.14. Ishikawa H, Imanishi K, Otani T, Okuda S, Tatsuta M, Ishiguro S. Effectiveness of endoscopic treatment of carcinoid tumors of the rectum. Endoscopy. 1989; 21:133–135.15. Son HJ, Sohn DK, Hong CW, Han KS, Kim BC, Park JW, Choi HS, Chang HJ, Oh JH. Factors associated with complete local excision of small rectal carcinoid tumor. Int J Colorectal Dis. 2013; 28:57–61.16. Cho SB, Park SY, Yoon KW, Lee S, Lee WS, Joo YE, Kim HS, Choi SK, Rew JS. The effect of post-biopsy scar on the submucosal elevation for endoscopic resection of rectal carcinoids. Korean J Gastroenterol. 2009; 53:36–42.17. Tichansky DS, Cagir B, Borrazzo E, Topham A, Palazzo J, Weaver EJ, Lange A, Fry RD. Risk of second cancers in patients with colorectal carcinoids. Dis Colon Rectum. 2002; 45:91–97.18. Choi CW, Kang DH, Kim HW, Park SB, Jo WS, Song GA, Cho M. Comparison of endoscopic resection therapies for rectal carcinoid tumor: endoscopic submucosal dissection versus endoscopic mucosal resection using band ligation. J Clin Gastroenterol. 2013; 47:432–436.19. Shim KN, Yang SK, Myung SJ, Chang HS, Jung SA, Choe JW, Lee YJ, Byeon JS, Lee JH, Jung HY, et al. Atypical endoscopic features of rectal carcinoids. Endoscopy. 2004; 36:313–316.20. Eggenberger JC. Carcinoid and other neuroendocrine tumors of the colon and rectum. Clin Colon Rectal Surg. 2011; 24:129–134.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Three Cases of Endoscopic Mucosal Resection of Rectal Carcinoid Tumor by Band Ligation and the Snare Resection Technique
- Tips and Tricks for Better Endoscopic Treatment of Colorectal Tumors: Usefulness of Cap and Band in Colorectal Endoscopic Mucosal Resection
- The Effect of Post-biopsy Scar on the Submucosal Elevation for Endoscopic Resection of Rectal Carcinoids
- Duodenal Carcinoid Tumor Treated by Endoscopic Mucosal Resection
- The Advantage of an Endoscopic Submucosal Tunneling Technique for Rectal Carcinoid Tumors