J Korean Med Sci.
2014 Mar;29(3):363-369. 10.3346/jkms.2014.29.3.363.
Intimal Hyperplasia in Loop-Injured Carotid Arteries Is Attenuated in Transglutaminase 2-Null Mice
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Biochemistry and Molecular Biology, Seoul National University College of Medicine, Seoul, Korea. igkim@snu.ac.kr
- KMID: 1734923
- DOI: http://doi.org/10.3346/jkms.2014.29.3.363
Abstract
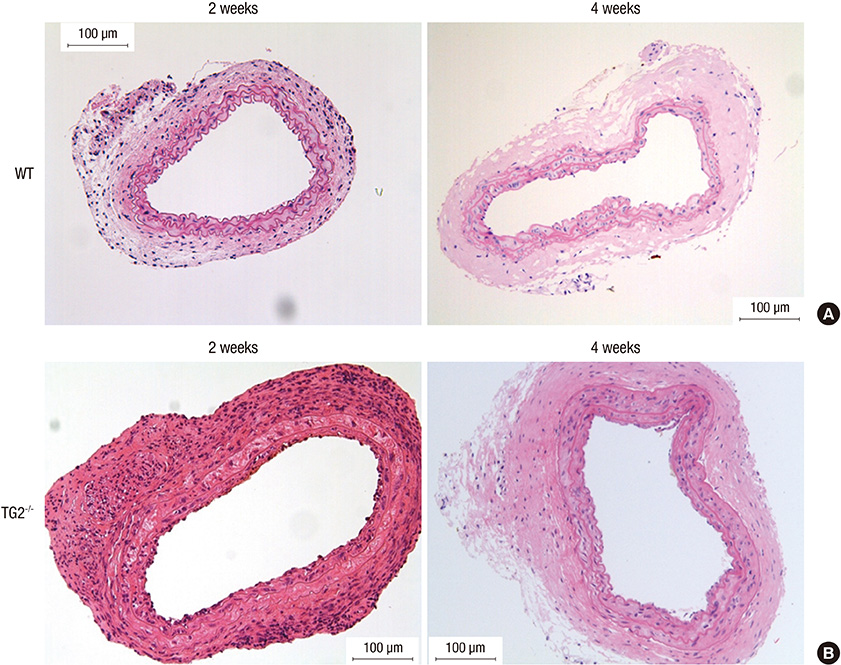
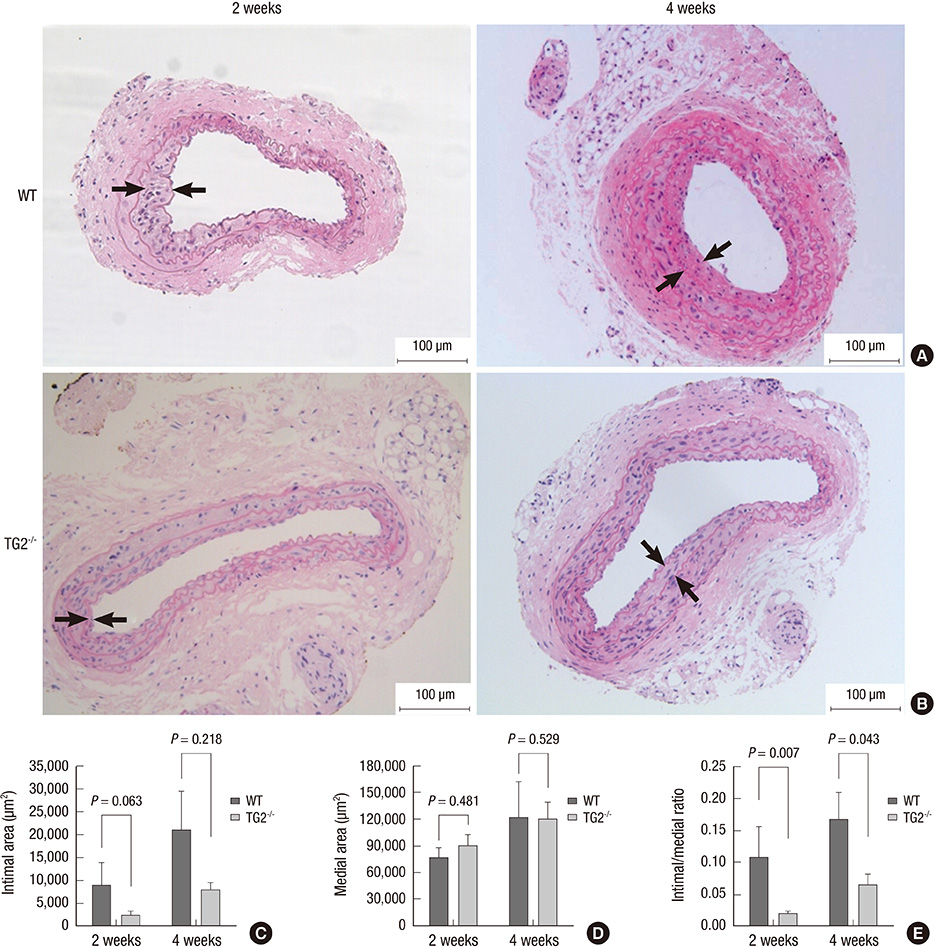
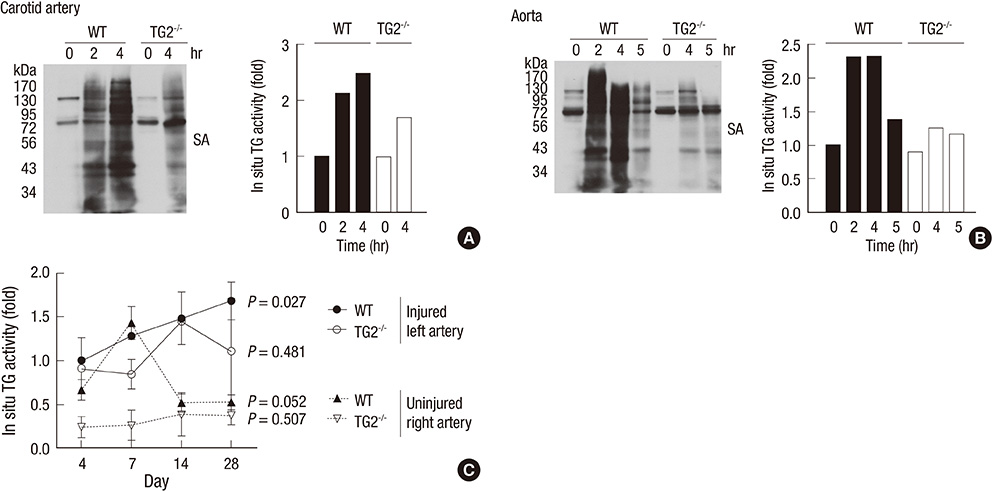
- Arterial restenosis frequently develops after open or endovascular surgery due to intimal hyperplasia. Since tissue transglutaminase (TG2) is known to involve in fibrosis, wound healing, and extracellular matrix remodeling, we examined the role of TG2 in the process of intimal hyperplasia using TG2-null mice. The neointimal formation was compared between TG2-null and wild-type (C57BL/6) mice by two different injury models; carotid ligation and carotid loop injury. In ligation model, there was no difference in intimal thickness between two groups. In loop injury model, intimal hyperplasia developed in both groups and the intimal/medial area ratio was significantly reduced in TG2-null mice (P = 0.007). TG2 was intensely stained in neointimal cells in 2 weeks. In situ activity of TG2 in the injured arteries steadily increased until 4 weeks compared to uninjured arteries. Taken together, intimal hyperplasia was significantly reduced in TG2-null mice, indicating that TG2 has an important role in the development of intimal hyperplasia. This suggests that TG2 may be a novel target to prevent the arterial restenosis after vascular surgery.
MeSH Terms
Figure
Reference
-
1. Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury: I. smooth muscle growth in the absence of endothelium. Lab Invest. 1983; 49:327–333.2. Mitra AK, Gangahar DM, Agrawal DK. Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia. Immunol Cell Biol. 2006; 84:115–124.3. Kester M, Waybill P, Kozak M. New strategies to prevent restenosis. Am J Cardiovasc Drugs. 2001; 1:77–83.4. Min SK, Kenagy RD, Clowes AW. Induction of vascular atrophy as a novel approach to treating restenosis: a review. J Vasc Surg. 2008; 47:662–670.5. Bauters C, Isner JM. The biology of restenosis. Prog Cardiovasc Dis. 1997; 40:107–116.6. Li W, Wang H, Kuang CY, Zhu JK, Yu Y, Qin ZX, Liu J, Huang L. An essential role for the Id1/PI3K/Akt/NFkB/survivin signalling pathway in promoting the proliferation of endothelial progenitor cells in vitro. Mol Cell Biochem. 2012; 363:135–145.7. Yoshimura S, Morishita R, Hayashi K, Yamamoto K, Nakagami H, Kaneda Y, Sakai N, Ogihara T. Inhibition of intimal hyperplasia after balloon injury in rat carotid artery model using cis-element 'decoy' of nuclear factor-kappaB binding site as a novel molecular strategy. Gene Ther. 2001; 8:1635–1642.8. Zubilewicz T, Wronski J, Bourriez A, Terlecki P, Guinault AM, Muscatelli-Groux B, Michalak J, Méllière D, Becquemin JP, Allaire E. Injury in vascular surgery--the intimal hyperplastic response. Med Sci Monit. 2001; 7:316–324.9. Iismaa SE, Mearns BM, Lorand L, Graham RM. Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol Rev. 2009; 89:991–1023.10. Zemskov EA, Janiak A, Hang J, Waghray A, Belkin AM. The role of tissue transglutaminase in cell-matrix interactions. Front Biosci. 2006; 11:1057–1076.11. Shin DM, Jeon JH, Kim CW, Cho SY, Lee HJ, Jang GY, Jeong EM, Lee DS, Kang JH, Melino G, et al. TGFbeta mediates activation of transglutaminase 2 in response to oxidative stress that leads to protein aggregation. FASEB J. 2008; 22:2498–2507.12. Oh K, Park HB, Byoun OJ, Shin DM, Jeong EM, Kim YW, Kim YS, Melino G, Kim IG, Lee DS. Epithelial transglutaminase 2 is needed for T cell interleukin-17 production and subsequent pulmonary inflammation and fibrosis in bleomycin-treated mice. J Exp Med. 2011; 208:1707–1719.13. Sohn J, Kim TI, Yoon YH, Kim JY, Kim SY. Novel transglutaminase inhibitors reverse the inflammation of allergic conjunctivitis. J Clin Invest. 2003; 111:121–128.14. Sane DC, Kontos JL, Greenberg CS. Roles of transglutaminases in cardiac and vascular diseases. Front Biosci. 2007; 12:2530–2545.15. Zhang Z, Vezza R, Plappert T, McNamara P, Lawson JA, Austin S, Praticò D, Sutton MS, FitzGerald GA. COX-2-dependent cardiac failure in Gh/tTG transgenic mice. Circ Res. 2003; 92:1153–1161.16. Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008; 102:529–537.17. Bakker EN, Buus CL, Spaan JA, Perree J, Ganga A, Rolf TM, Sorop O, Bramsen LH, Mulvany MJ, Vanbavel E. Small artery remodeling depends on tissue-type transglutaminase. Circ Res. 2005; 96:119–126.18. Bakker EN, Pistea A, Spaan JA, Rolf T, de Vries CJ, van Rooijen N, Candi E, VanBavel E. Flow-dependent remodeling of small arteries in mice deficient for tissue-type transglutaminase: possible compensation by macrophage-derived factor XIII. Circ Res. 2006; 99:86–92.19. De Laurenzi V, Melino G. Gene disruption of tissue transglutaminase. Mol Cell Biol. 2001; 21:148–155.20. Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997; 17:2238–2244.21. Cho A, Reidy MA. Matrix metalloproteinase-9 is necessary for the regulation of smooth muscle cell replication and migration after arterial injury. Circ Res. 2002; 91:845–851.22. Jeon JH, Choi KH, Cho SY, Kim CW, Shin DM, Kwon JC, Song KY, Park SC, Kim IG. Transglutaminase 2 inhibits Rb binding of human papillomavirus E7 by incorporating polyamine. EMBO J. 2003; 22:5273–5282.23. Jeong EM, Kim CW, Cho SY, Jang GY, Shin DM, Jeon JH, Kim IG. Degradation of transglutaminase 2 by calcium-mediated ubiquitination responding to high oxidative stress. FEBS Lett. 2009; 583:648–654.24. Shin DM, Jeon JH, Kim CW, Cho SY, Kwon JC, Lee HJ, Choi KH, Park SC, Kim IG. Cell type-specific activation of intracellular transglutaminase 2 by oxidative stress or ultraviolet irradiation: implications of transglutaminase 2 in age-related cataractogenesis. J Biol Chem. 2004; 279:15032–15039.25. Lindner V, Fingerle J, Reidy MA. Mouse model of arterial injury. Circ Res. 1993; 73:792–796.26. Fesus L, Piacentini M. Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem Sci. 2002; 27:534–539.27. Jang GY, Jeon JH, Cho SY, Shin DM, Kim CW, Jeong EM, Bae HC, Kim TW, Lee SH, Choi Y, et al. Transglutaminase 2 suppresses apoptosis by modulating caspase 3 and NF-kappaB activity in hypoxic tumor cells. Oncogene. 2010; 29:356–367.28. Facchiano F, Facchiano A, Facchiano AM. The role of transglutaminase-2 and its substrates in human diseases. Front Biosci. 2006; 11:1758–1773.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of intimal hyperplasia by local perivascular application of rapamycin and imatinib mesilate after carotid balloon injury
- Anastomotic Intimal Hyperplasia and TGF-beta1 mRNA Expression after a PTFE Graft in a Rabbit Carotid Artery
- Different Responses of Neointimal Cells to Imatinib Mesylate and Rapamycin Compared with Normal Vascular Smooth Muscle Cells
- The Effect of Epigallocatechin-3-Gallate on Intimal Hyperplasia after Vascular Grafting
- Relationship of Serum Ferritin, Cholesterol, and Intimal Hyperplasia after Mechanical Injury to Carotid Artery in a Rat Model