Yonsei Med J.
2013 May;54(3):650-657. 10.3349/ymj.2013.54.3.650.
ROS1 Expression in Invasive Ductal Carcinoma of the Breast Related to Proliferation Activity
- Affiliations
-
- 1Department of Pathology, Yonsei University Wonju College of Medicine, Wonju, Korea. abba@yonsei.ac.kr
- 2Department of Occupational and Environmental Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 3Department of Surgery, Yonsei University Wonju College of Medicine, Wonju, Korea.
- KMID: 1727878
- DOI: http://doi.org/10.3349/ymj.2013.54.3.650
Abstract
- PURPOSE
ROS1 is an oncogene, expressed primarily in glioblastomas of the brain that has been hypothesized to mediate the effects of early stage tumor progression. In addition, it was reported that ROS1 expression was observed in diverse cancer tissue or cell lines and ROS1 is associated with the development of several tumors. However, ROS1 expression has not been studied in breast cancer to date. Therefore, we investigated ROS1 expression at the protein and gene level to compare expression patterns and to verify the association with prognostic factors in invasive ductal carcinoma (IDC) of the breast.
MATERIALS AND METHODS
Tissue samples from 203 patients were used. Forty-six cases were available for fresh tissue. We performed immunohistochemical staining and real-time polymerase chain reaction (PCR).
RESULTS
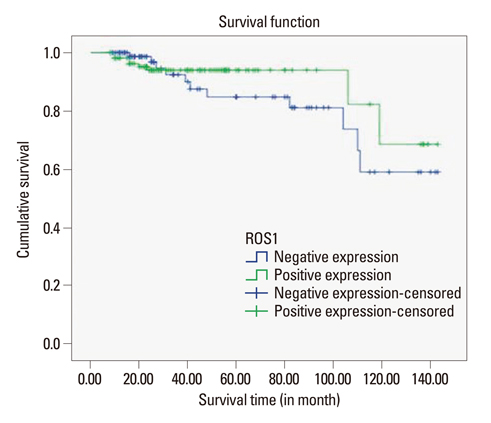
ROS1 expression was significantly lower in proportion to higher histologic grade, higher mitotic counts, lower estrogen receptor expression, and a higher Ki-67 proliferation index, although ROS1 expression was not significantly associated with the survival rate. The result of real-time PCR revealed similar trends, however not statistically significant.
CONCLUSION
Higher ROS1 expression may be associated with favorable prognostic factors of IDC and its expression in IDC is related to the proliferation of tumor cells.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Breast Neoplasms/*metabolism/pathology
Carcinoma, Ductal, Breast/*metabolism/pathology
Cell Proliferation
Female
Humans
Immunohistochemistry
Middle Aged
Neoplasm Grading
Prognosis
Protein-Tyrosine Kinases/genetics/*metabolism
Proto-Oncogene Proteins/genetics/*metabolism
Survival Analysis
Proto-Oncogene Proteins
Protein-Tyrosine Kinases
Figure
Reference
-
1. Birchmeier C, O'Neill K, Riggs M, Wigler M. Characterization of ROS1 cDNA from a human glioblastoma cell line. Proc Natl Acad Sci U S A. 1990. 87:4799–4803.
Article2. Chen J, Zong CS, Wang LH. Tissue and epithelial cell-specific expression of chicken proto-oncogene c-ros in several organs suggests that it may play roles in their development and mature functions. Oncogene. 1994. 9:773–780.3. Acquaviva J, Wong R, Charest A. The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer. Biochim Biophys Acta. 2009. 1795:37–52.
Article4. Eom M, Han A, Yi SY, Shin JJ, Cui Y, Park KH. RHEB expression in fibroadenomas of the breast. Pathol Int. 2008. 58:226–232.
Article5. Zhao JF, Sharma S. Expression of the ROS1 oncogene for tyrosine receptor kinase in adult human meningiomas. Cancer Genet Cytogenet. 1995. 83:148–154.
Article6. Bonner AE, Lemon WJ, Devereux TR, Lubet RA, You M. Molecular profiling of mouse lung tumors: association with tumor progression, lung development, and human lung adenocarcinomas. Oncogene. 2004. 23:1166–1176.
Article7. Yovchev MI, Grozdanov PN, Joseph B, Gupta S, Dabeva MD. Novel hepatic progenitor cell surface markers in the adult rat liver. Hepatology. 2007. 45:139–149.
Article8. Ruhe JE, Streit S, Hart S, Wong CH, Specht K, Knyazev P, et al. Genetic alterations in the tyrosine kinase transcriptome of human cancer cell lines. Cancer Res. 2007. 67:11368–11376.
Article9. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991. 19:403–410.
Article10. Tavassoli FA, Devilee P. Pathology and genetics of tumours of the breast and female genital organs. 2003. Lyon: IARC Press.11. Kumar B, De Silva M, Venter DJ, Armes JE. Tissue microarrays: a practical guide. Pathology. 2004. 36:295–300.
Article12. Park KH, Choi SE, Eom M, Kang Y. Downregulation of the anaphase-promoting complex (APC)7 in invasive ductal carcinomas of the breast and its clinicopathologic relationships. Breast Cancer Res. 2005. 7:R238–R247.
Article13. Dabbs DJ. Diagnostic immunohistochemistry: theranostic and genomic applications. 2010. 3rd ed. Philadelphia (PA): Saunders/Elsevier.14. Birchmeier C, Sharma S, Wigler M. Expression and rearrangement of the ROS1 gene in human glioblastoma cells. Proc Natl Acad Sci U S A. 1987. 84:9270–9274.
Article15. Charest A, Kheifets V, Park J, Lane K, McMahon K, Nutt CL, et al. Oncogenic targeting of an activated tyrosine kinase to the Golgi apparatus in a glioblastoma. Proc Natl Acad Sci U S A. 2003. 100:916–921.
Article16. Charest A, Lane K, McMahon K, Park J, Preisinger E, Conroy H, et al. Fusion of FIG to the receptor tyrosine kinase ROS in a glioblastoma with an interstitial del(6)(q21q21). Genes Chromosomes Cancer. 2003. 37:58–71.
Article17. Gu TL, Deng X, Huang F, Tucker M, Crosby K, Rimkunas V, et al. Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma. PLoS One. 2011. 6:e15640.
Article18. Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007. 131:1190–1203.
Article19. Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012. 30:863–870.20. Rosen PP. Rosen's breast pathology. 2009. 3rd ed. Philadelphia (PA): Wolters Kluwer/Lippincott Williams & Wilkins.21. Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran's pathologic basis of disease. 2010. 8th ed. Philadelphia (PA): Saunders.22. Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957. 11:359–377.
Article23. Meyer JS, Alvarez C, Milikowski C, Olson N, Russo I, Russo J, et al. Breast carcinoma malignancy grading by Bloom-Richardson system vs proliferation index: reproducibility of grade and advantages of proliferation index. Mod Pathol. 2005. 18:1067–1078.
Article24. van Diest PJ, van der Wall E, Baak JP. Prognostic value of proliferation in invasive breast cancer: a review. J Clin Pathol. 2004. 57:675–681.
Article25. Trihia H, Murray S, Price K, Gelber RD, Golouh R, Goldhirsch A, et al. Ki-67 expression in breast carcinoma: its association with grading systems, clinical parameters, and other prognostic factors--a surrogate marker? Cancer. 2003. 97:1321–1331.26. Miglietta L, Vanella P, Canobbio L, Naso C, Cerisola N, Meszaros P, et al. Prognostic value of estrogen receptor and Ki-67 index after neoadjuvant chemotherapy in locally advanced breast cancer expressing high levels of proliferation at diagnosis. Oncology. 2010. 79:255–261.
Article27. Yasuda H, de Figueiredo-Pontes LL, Kobayashi S, Costa DB. Preclinical rationale for use of the clinically available multitargeted tyrosine kinase inhibitor crizotinib in ROS1-translocated lung cancer. J Thorac Oncol. 2012. 7:1086–1090.
Article28. Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, Hatano S, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012. 18:378–381.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Uncoupling Protein 2 (UCP2) and p53 Expression in Invasive Ductal Carcinoma of Breast
- Expression of MT-1 MMP, MMP2, MMP9 and TIMP2 mRNAs in Ductal Carcinoma in Situ and Invasive Ductal Carcinoma of the Breast
- HDAC1 Expression in Invasive Ductal Carcinoma of the Breast and Its Value as a Good Prognostic Factor
- The Galectin 3 Expression in Benign and Malignant Breast Tumor
- Invasive Ductal Carcinoma of the Male Breast: A Case Report and Review of the Literature