Tuberc Respir Dis.
2006 Apr;60(4):437-450. 10.4046/trd.2006.60.4.437.
Pretreatment of Diltiazem Ameliorates Endotoxin-Induced Acute Lung Injury by Suppression of Neutrophilic Oxidative Stress
- Affiliations
-
- 1Department of Physiology, School of Medicine, Daegu Catholic University, Daegu, Korea. hdsomn@cu.ac.kr
- 2Department of Chest Surgery, School of Medicine, Daegu Catholic University, Daegu, Korea.
- 3Department of Internal Medicine, School of Medicine, Daegu Catholic University, Daegu, Korea.
- KMID: 1719369
- DOI: http://doi.org/10.4046/trd.2006.60.4.437
Abstract
-
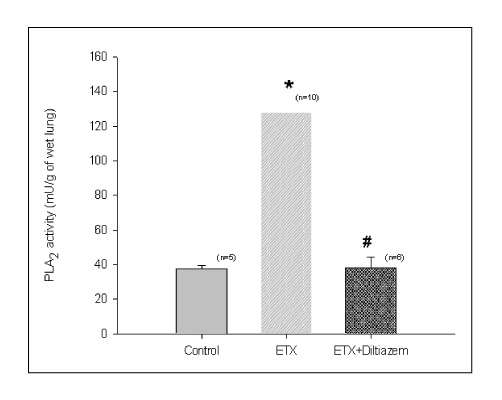
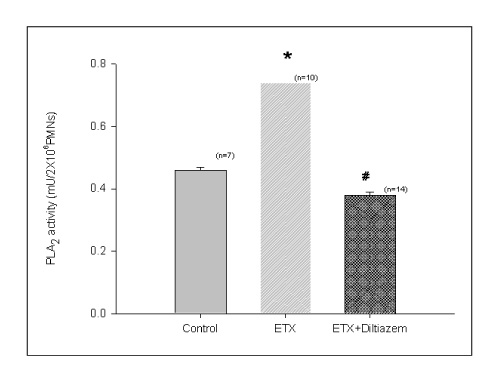
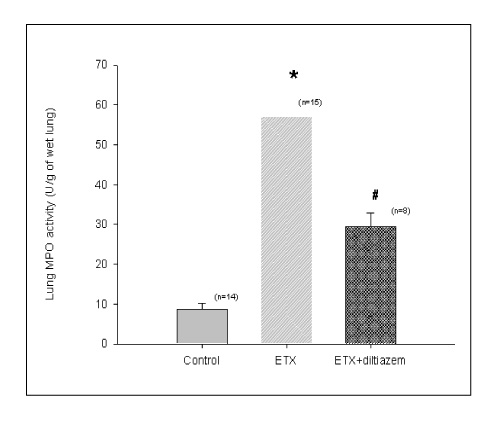
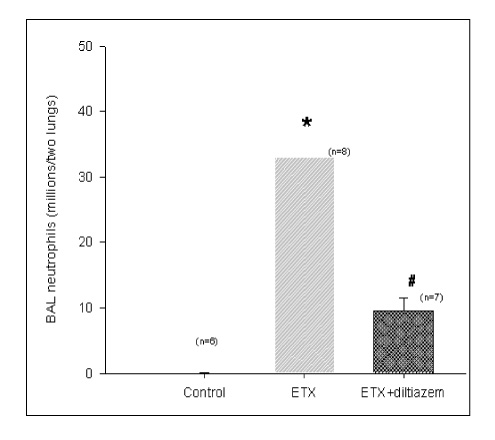
BACKGROUND: Acute respiratory distress syndrome (ARDS) is characterized by severe inflammatory pulmonary edema of unknown pathogenesis. To investigate the pathogenesis of ARDS associated with neutrophilic oxidative stress, the role of phospholipase A2 (PLA2) was evaluated by the inhibition of calcium channel.
METHODS
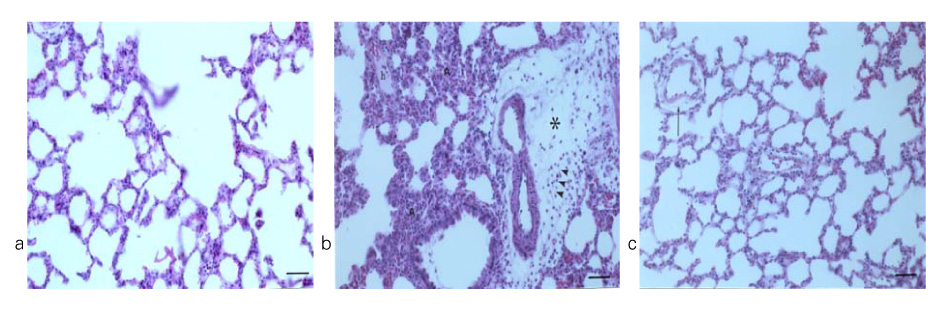
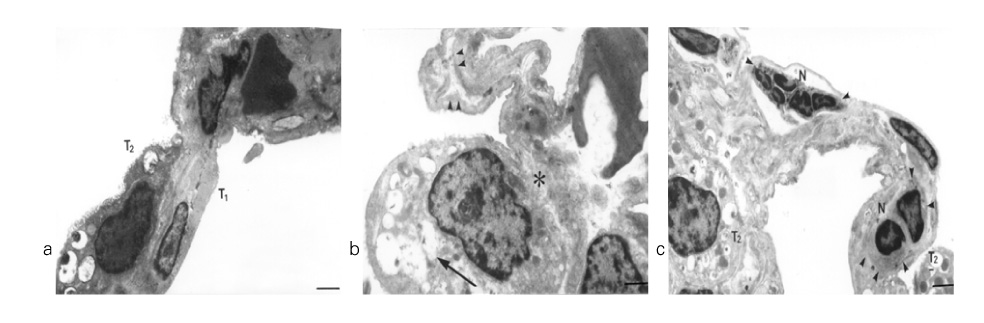
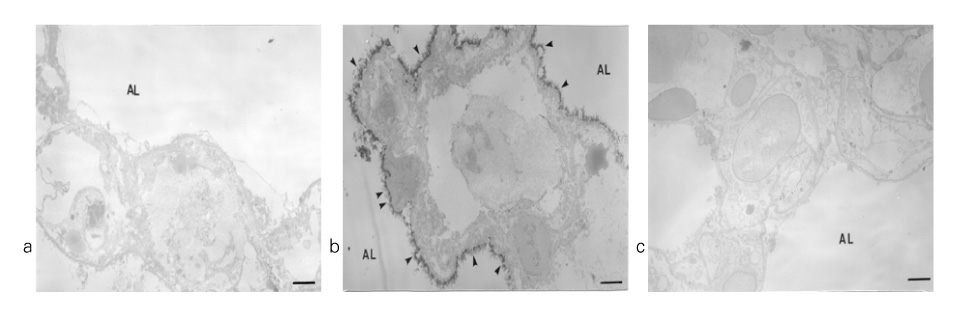
In Sprague-Dawley rats, acute lung injury (ALI) was induced by the instillation of E.coli endotoxin (ETX) into the trachea. At the same time, diltiazem was given 60 min prior to tracheal instillation of ETX. Parameters of ALI such as lung and neutrophil PLA2, lung myeloperoxidase (MPO), BAL neutrophils, protein, surfactant were measured. Production of free radicals from neutrophils was measured also. Morphological studies with light microscope and electron microscope were carried out and electron microscopic cytochemistry for detection of free radicals was performed also.
RESULTS
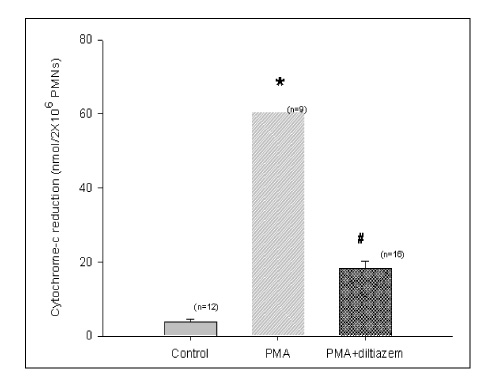
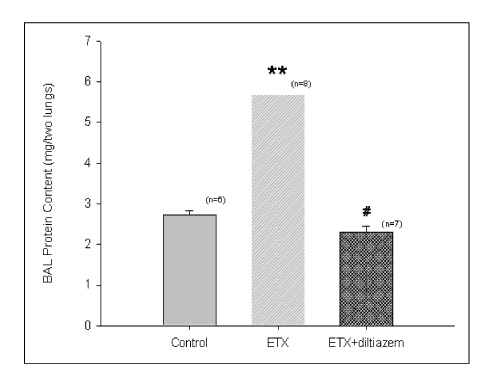
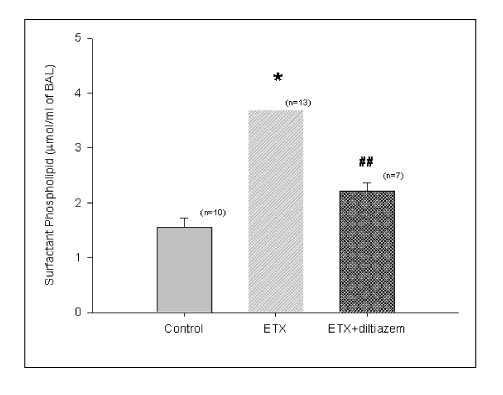
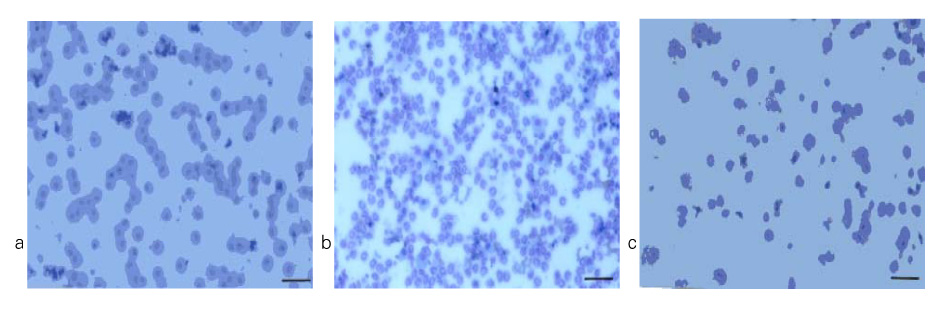
Diltiazem had decreased the ALI parameters effectively in ETX given rats and decreased the production of free radicals from neutrophils and lung tissues. Morphological studies denoted the protective effects of diltiazem.
CONCLUSION
Diltiazem, a calcium channel blocker, was effective in amelioration of ALI by the suppression of neutrophilic oxidative stress mediated by PLA2 activation.
Keyword
MeSH Terms
Figure
Reference
-
1. Ashbaugh DG, Bigelow DB, Petty PL, Levine BE. Acute respiratory distress in adults. Lancet. 1967. 2:319–323.2. Connelly KG, Repine JE. Markers for predicting the development of acute respiratory distress syndrome. Ann Rev Med. 1997. 48:429–445.3. Martensson J, Jain A, Frayer W, Meister A. Glutathione metabolism in the lung : inhibition of its synthesis leads to lamellar body and mitochondrial defects. Proc Natl Acad Sci. 1989. 86:5296–5300.4. Matthay MA. Conference summary : Acute lung injury. Chest. 1999. 116:119S–126S.5. Fujishima S, Aikawa N. Neutrophil-mediated tissue injury and its modulation. Int Care Med. 1995. 21:227–285.6. Pittet JF, Mackersie RC, Martin TR, Matthay MA. Biological markers of acute lung injury : prognostic and pathogenetic significance. Am J Respir Crit Care Med. 1997. 155:1187–1205.7. Pruzanski W, Vadas P. Phospholipase A2-a mediator between proximal and distal effectors of inflammation. Immunol Today. 1991. 12:143–146.8. Koh T, Hybertson BM, Jepson EK, Cho OJ, Repine JE. Cytokine induced neutrophil chemoattratant is necessary for interleukin-1 induced lung leak in rats. J Appl Physiol. 1995. 79:472–478.9. Hybertson BM, Jepson DK, Allard JD, Cho OJ, Lee YM, Huddleston JR, Weinman JP, Oliva AM, Repine JE. Transforming growth factorβ contributes to lung leak in rats given interleukin-1 intracheally. Exp Lung Res. 2003. 29:361–373.10. Lee YM, Hypertson BM, Terada LS, Repine AJ, Cho HG, Repine JE. Mepacrine decreases lung leak in rats given interleukin-1 intratracheally. Am J Respir Crit Care Med. 1997. 155:1624–1628.11. Lee YM, Hybertson BM, Cho HG, Terda LS, Repine AJ, Repine JE. Platelet-activating factor contributes to acute lung leak in rats given interleukin-1 intratracheally. Am J Physiol (Lung Cell Mol Physiol). 2000. 279:L75–L80.12. Lee YM, Park Y. PAF contributes to intestinal ischemia reperfusion induced acute lung injury through neutrophilic oxidative stress. Korean J Physiol Pharmacol. 1999. 3:405–414.13. Canham EM, Shoemaker SA, Tate RM, Harada RM, McMurtry IF, Repine JE. Mepacrine but not methylprednisolone decreases acute edematous lung injury after injection of phorbol myristate acetate in rabbits. Am Rev Respir Dis. 1983. 127:594–598.14. Crystal RG, West JB, Weibel ER, Barnes PJ. Chapter 4 ; glucocoid receptors. The lung. 1997. Vol 1. Lippincott Raven: Mew York, Philadelphia;45.15. Viejo A, Goodyear-Bruch C, Pierce JD. Oxidative stress in critically ill patients. Am J Crit Care. 2002. 11:543–551.16. Partrick DA, Moore EE, Silliman CC, Barnett CC, Kuypers FA. Secretory PLA2 activity correlates with postinjury multiple organ failure. Crit Care Med. 2001. 29:42–46.17. Nagase T, Uozumi N, Ishii S, Kume K, Izumi T, Ouchi Y, Shimizu T. Acute lung injury by sepsis and acid aspiration : a key role for cytosolic phospholipase A2. Nature Immunol. 2000. 1:42–46.18. Vernon LP, Bell JD. Membrane structure, toxins and phospholipase A2 activity. Pharmac Ther. 1992. 54:269–295.19. Kwon YS. Changes in phospholipase A2 activity in the pulmonary oxidative stress of the platelet-activating factor-induced acute lung injury. 2002. Daegu catholic University;Doctoral Thesis.20. Katsumata M, Gupta C, Goldman AS. Rapid assay for activity of phospholipase A2 using radioactive substrate. Anal Biochem. 1986. 154:676–681.21. Haslett C, Guthrie LA, Kopaniak MM, Johnston JrBB, Henson PM. Modulation of multiple neutrophil functions by preparative methods or trace concentration of bacterial lipopolysaccharides. Am J Pathol. 1985. 119:101–110.22. Goldblum SE, Wu KE, Jay M. Lung myeloperoxidase as a measurement of leukostasis in rabbits. J Appl Physiol. 1985. 59:1978–1985.23. Botha AJ, Moore FA, Moore EE, Fontes B, Banerjee A, Peterson VM. Post injury neutrophil priming and activation state : therapeutic challenges. Shock. 1995. 3:537–566.24. Brown RE, Jarvis KL, Hyland KJ. Protein measurement using bicinchoninic acid : elimination of interfering substance. Anal Biochem. 1989. 180:136–139.25. Bligh EG, Dyer WJ. A rapid method of lipid extraction and purification. Can J Biochem Physiol. 1959. 37:911–917.26. Hess HH, Derr JE. Assay of inorganic and organic phosphorus in the 0.1-5 nanomolar range. Anal Biochem. 1975. 63:607–613.27. Corbet A, Creegan J, Frink J, Rudolph AJ. Distension produced phospholipid secertion in postmortem in situ lungs of normal rabbits. Am Rev Respir Dis. 1983. 128:695–701.28. Hobson J, Wright J, Churg A. Histochemical evidence for generation of active oxygen species on the apical surface of cigarette smoke exposed tracheal explants. Am J Pathol. 1991. 139:573–580.29. Pruzanski W, Vadas P, Browing J. Secretory non-pancreatic groupIIphospholipase A2 : role in physiologic and inflammatory process. J Lipid Mediat. 1993. 8:161–167.30. Lee YM, Hybertson BM, Cho HG, Repine JE. Platelet-activating factor induces lung inflammation and leak in rats: Hydrogen peroxide production along neutrophil-lung endothelial cell interfaces. J Lab Clin Med. 2002. 140:312–319.31. Sun X, Caplan MS, Hsueh W. Tumor necrosis factor and endotoxin synergistically activate intestinal phospholipase A2 in mice. Role of endogenous platelet activating factor and effect of exogenous platelet activating factor. Gut. 1994. 35:215–219.32. Kramer RM, Sharp JD. Structure, function and regulation of Ca2+-Sensitive cytosolic phospholipase A2 (PLA2). FEBS Letters. 1997. 410:49–53.33. Nakashima S, Suganuma A, Sato M, Tohmatsu T, Nozawa Y. Mechanism of arachidonic acid liberation in platelet-activating factor stimulated human polymorphonuclear neutrophils. J Immunol. 1989. 143:1295–1302.34. Bomalaski JS, Baker DG, Brophy L, Resurrecion NV, Spilberg I, Muniain M, Clark MA. A phospholipase A2 activating protein (PLAP) stimulates human neutrophil aggregation and release of lysosomal enzymes, superoxide, and eicosanoids. J Immunol. 1989. 142:3957–3962.35. Koike K, Moore EE, Moore FA, Kim FJW, Carl VS, Banerjee A. Gut Phospholipase A2 mediates neutrophil priming and lung injury after mesenteric ischemia-reperfusion. Am J Physiol. 1995. 268:G397–G403.36. Dana R, Leto TL, Levy R. Essential requirement of cytosolic phospholipase A2 for activation of the phagocyte NADPH oxidase. J Biol Chem. 1998. 273:441–445.37. Delclaux C, Rzaiguia-Delclaux S, Delacourt C, Brun-Buisson C, Lafuma C, Harf A. Alveolar neutrophils in endotoxin-induced and bacteria-induced acute lung injury in rat. Am J Physiol. 1997. 273:L104–L112.38. Roh WS, Koo BU, Lee YM. Inhibition of phospholipase A2 ameliorates the acute lung injury induced by E coli endotoxin via reduced production of oxygen free radicals in the lung. Korean J Anethesiol. 2001. 41:86–97.39. Tsukahara Y, Morisaki T, Horita Y, Torisu M, Tanaka M. Pholpholipase A2 mediates nitric oxide production by alveolar macrophages and acute lung injury in pancreatitis. Ann Surg. 1999. 229:385–392.40. Nakos G, Kitsiouli EI, Tsangaris I, Lekka ME. Bronchoalveloar lavage fluid characteristic of early intermediate and late phase of ARDS. Alterations in leukocytes, proteins, PAF and surfactant components. Intensive Care Med. 1998. 24:296–303.41. Martensson J, Jain A, Stole E, Frayer W, Auld PAM, Meister A. Inhibition of glutathione synthesis in the newborn rabbit : A model for endogenously produced oxidative stress. Proc Natl Acad Sci. 1991. 88:9360–9364.42. McIntosh JC, Swyers AH, Fisher JH, Wright JR. Surfactant proteins A and D increase in response to intratracheal lipopolysaccharide. Am Respir Cell Mol Biol. 1996. 15:509–519.43. Lee YM, Park Y, Koh Y. Effects of high dose of dexamethasone on PLA2, GGT activity and lung morphology in NNNMU-induced ARDS rats. Tuberculosis Respir Dis. 1996. 43:925–935.44. Ortolani O, Conti A, De Gaudio AR, Masoni M, Novelli G. Protective effects of N-acetylcysteine and rutin on the lipid peroxidation on the lung epithelium during the adult respiratory distress syndrome. Shock. 2000. 13:14–18.45. Koike K, Yamamoto Y, Hori Y, Ono T. Group II phospholipase A2 mediates lung injury in intestinal ischemia-reperfusion. Ann Surg. 2000. 232:90–97.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of the PLA2-Activated Neutrophilic Oxidative Stress in Oleic Acid-Induced Acute Lung Injury
- Effect of the inhibition of PLA2 on the oxidative stress in the lungs of glutathione depleted rats given endotoxin intratracheally
- Presumptive Role of Neutrophilic Oxidative Stress in Oxygen-induced Acute Lung Injury in Rats
- Phospholipase A2 Contributes to Hemorrhage-induced Acute Lung Injury Through Neutrophilic Respiratory Burst
- Inhibition of Phospholipase A2 Ameliorates the Acute Lung Injury Induced by E Coli Endotoxin via Reduced Production of Oxygen Free Radicals in the Lung