J Vet Sci.
2013 Sep;14(3):329-335. 10.4142/jvs.2013.14.3.329.
Improved rat spinal cord injury model using spinal cord compression by percutaneous method
- Affiliations
-
- 1Department of Veterinary Surgery, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea. hykim@konkuk.ac.kr
- 2Department of Veterinary Surgery, College of Veterinary Medicine, Gyeongsang National University, Jinju 660-701, Korea.
- 3School of Veterinary Medicine, University of California, Davis, CA 95616, USA.
- 4Seoul Cord Blood Bank, Histostem Co., Seongnam 462-807, Korea.
- 5Department of Veterinary Clinical Pathology, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea.
- KMID: 1705564
- DOI: http://doi.org/10.4142/jvs.2013.14.3.329
Abstract
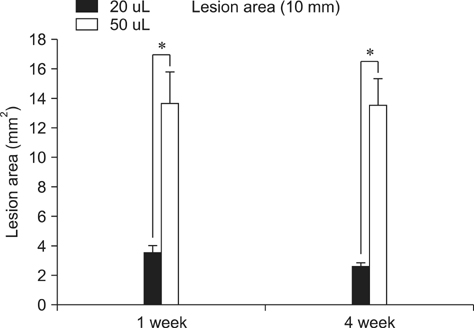
- Here, percutaneous spinal cord injury (SCI) methods using a balloon catheter in adult rats are described. A balloon catheter was inserted into the epidural space through the lumbosacral junction and then inflated between T9-T10 for 10min under fluoroscopic guidance. Animals were divided into three groups with respect to inflation volume: 20 microL (n = 18), 50 microL (n = 18) and control (Fogarty catheter inserted but not inflated; n = 10). Neurological assessments were then made based on BBB score, magnetic resonance imaging and histopathology. Both inflation volumes produced complete paralysis. Gradual recovery of motor function occurred when 20 microL was used, but not after 50 microL was applied. In the 50 microL group, all gray and white matter was lost from the center of the lesion. In addition, supramaximal damage was noted, which likely prevented spontaneous recovery. This percutaneous spinal cord compression injury model is simple, rapid with high reproducibility and the potential to serve as a useful tool for investigation of pathophysiology and possible protective treatments of SCI in vivo.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Expression of neurotrophic factors in injured spinal cord after transplantation of human-umbilical cord blood stem cells in rats
Hyo-jin Chung, Wook-hun Chung, Jae-Hoon Lee, Dai-Jung Chung, Wo-Jong Yang, A-Jin Lee, Chi-Bong Choi, Hwa-Seok Chang, Dae-Hyun Kim, Hyun Jung Suh, Dong-Hun Lee, Soo-Han Hwang, Sun Hee Do, Hwi-Yool Kim
J Vet Sci. 2016;17(1):97-102. doi: 10.4142/jvs.2016.17.1.97.
Reference
-
1. Aoki M, Kishima H, Yoshimura K, Ishihara M, Ueno M, Hata K, Yamashita T, Iwatsuki K, Yoshimine T. Limited functional recovery in rats with complete spinal cord injury after transplantation of whole-layer olfactory mucosa. J Neurosurg Spine. 2010; 12:122–130.
Article2. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995; 12:1–21.
Article3. Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996; 139:244–256.
Article4. Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ, Nockels R, Perot PL, Salzman SK, Young W. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. J Neurotrauma. 1996; 13:343–359.
Article5. Behrmann DL, Bresnahan JC, Beattie MS. Modeling of acute spinal cord injury in the rat: neuroprotection and enhanced recovery with methylprednisolone, U-74006F and YM-14673. Exp Neurol. 1994; 126:61–75.
Article6. Benzel EC, Lancon JA, Bairnsfather S, Kesterson L. Effect of dosage and timing of administration of naloxone on outcome in the rat ventral compression model of spinal cord injury. Neurosurgery. 1990; 27:597–601.
Article7. Bilgen M, Abbe R, Narayana PA. Dynamic contrastenhanced MRI of experimental spinal cord injury: in vivo serial studies. Magn Reson Med. 2001; 45:614–622.
Article8. Blight AR. Spinal cord injury models: Neurophysiology. J Neurotrauma. 1992; 9:147–150.
Article9. Bresnahan JC, Beattie MS, Todd FD 3rd, Noyes DH. A behavioral and anatomical analysis of spinal cord injury produced by a feedback-controlled impaction device. Exp Neurol. 1987; 95:548–570.
Article10. Fehlings MG, Tator CH. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp Neurol. 1995; 132:220–228.
Article11. Fukuda S, Nakamura T, Kishigami Y, Endo K, Azuma T, Fujikawa T, Tsutsumi S, Shimizu Y. New canine spinal cord injury model free from laminectomy. Brain Res Brain Res Protoc. 2005; 14:171–180.
Article12. Khan M, Griebel R. Acute spinal cord injury in the rat: comparison of three experimental techniques. Can J Neurol Sci. 1983; 10:161–165.
Article13. Konno S, Yabuki S, Sato K, Olmarker K, Kikuchi S. A model for acute, chronic, and delayed graded compression of the dog cauda equina: presentation of the gross, microscopic, and vascular anatomy of the dog cauda equina and accuracy in pressure transmission of the compression model. Spine (Phila Pa 1976). 1995; 20:2758–2764.
Article14. Kwon BK, Oxland TR, Tetzlaff W. Animal models used in spinal cord regeneration research. Spine (Phila Pa 1976). 2002; 27:1504–1510.
Article15. Lee JH, Choi CB, Chung DJ, Kang EH, Chang HS, Hwang SH, Han H, Choe BY, Sur JH, Lee SY, Kim HY. Development of an improved canine model of percutaneous spinal cord compression injury by balloon catheter. J Neurosci Methods. 2008; 167:310–316.
Article16. Lim JH, Jung CS, Byeon YE, Kim WH, Yoon JH, Kang KS, Kweon OK. Establishment of a canine spinal cord injury model induced by epidural balloon compression. J Vet Sci. 2007; 8:89–94.
Article17. Lonjon N, Kouyoumdjian P, Prieto M, Bauchet L, Haton H, Gaviria M, Privat A, Perrin FE. Early functional outcomes and histological analysis after spinal cord compression injury in rats. J Neurosurg Spine. 2010; 12:106–113.
Article18. Martin D, Schoenen J, Delrée P, Gilson V, Rogister B, Leprince P, Stevenaert A, Moonen G. Experimental acute traumatic injury of the adult rat spinal cord by a subdural inflatable balloon: methodology, behavioral analysis, and histopathology. J Neurosci Res. 1992; 32:539–550.
Article19. Metz GA, Curt A, van de Meent H, Klusman I, Schwab ME, Dietz V. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J Neurotrauma. 2000; 17:1–17.
Article20. Pedram MS, Dehghan MM, Soleimani M, Sharifi D, Marjanmehr SH, Nasiri Z. Transplantation of a combination of autologous neural differentiated and undifferentiated mesenchymal stem cells into injured spinal cord of rats. Spinal Cord. 2010; 48:457–463.
Article21. Poon PC, Gupta D, Shoichet MS, Tator CH. Clip compression model is useful for thoracic spinal cord injuries: histologic and functional correlates. Spine (Phila Pa 1976). 2007; 32:2853–2859.22. Purdy PD, Duong RT, White CL 3rd, Baer DL, Reichard RR, Pride GL Jr, Adams C, Miller S, Hladik CL, Yetkin Z. Percutaneous translumbar spinal cord compression injury in a dog model that uses angioplasty balloons: MR imaging and histopathologic findings. AJNR Am J Neuroradiol. 2003; 24:177–184.23. Rivlin AS, Tator CH. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg Neurol. 1978; 10:38–43.24. Rosenzweig ES, McDonald JW. Rodent models for treatment of spinal cord injury: research trends and progress toward useful repair. Curr Opin Neurol. 2004; 17:121–131.
Article25. Sato K, Konno S, Yabuki S, Mao GP, Olmarker K, Kikuchi S. A model for acute, chronic, and delayed graded compression of the dog cauda equina: neurophysiologic and histologic changes induced by acute, graded compression. Spine (Phila Pa 1976). 1995; 20:2386–2391.
Article26. Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE Jr. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003; 20:179–193.
Article27. Vanický I, Urdzíková L, Saganová K, Cízková D, Gálik J. A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J Neurotrauma. 2001; 18:1399–1407.
Article28. Wrathall JR, Pettegrew RK, Harvey F. Spinal cord contusion in the rat: production of graded, reproducible, injury groups. Exp Neurol. 1985; 88:108–122.
Article29. Zhang Z, Nonaka H, Nagayama T, Hatori T, Ihara F, Zhang L, Akima M. Circulatory disturbance of rat spinal cord induced by occluding ligation of the dorsal spinal vein. Acta Neuropathol. 2001; 102:335–338.
Article