J Vet Sci.
2013 Jun;14(2):135-141. 10.4142/jvs.2013.14.2.135.
Antiviral effect of dietary germanium biotite supplementation in pigs experimentally infected with porcine reproductive and respiratory syndrome virus
- Affiliations
-
- 1College of Veterinary Medicine, Chonnam National University, Gwangju 500-757, Korea. bjlee@chonnam.ac.kr
- KMID: 1705514
- DOI: http://doi.org/10.4142/jvs.2013.14.2.135
Abstract
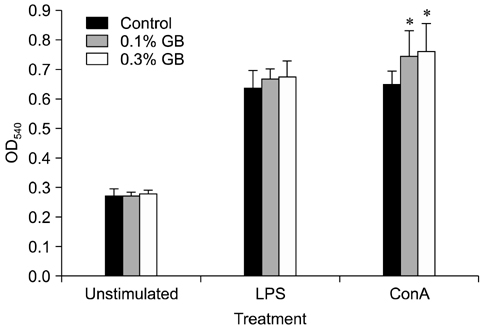
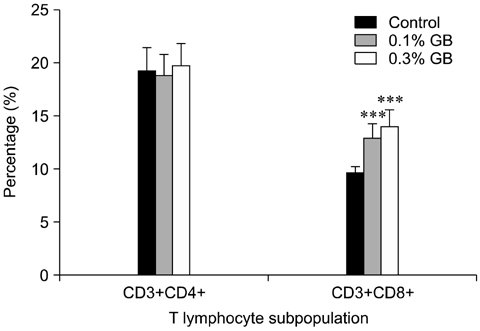
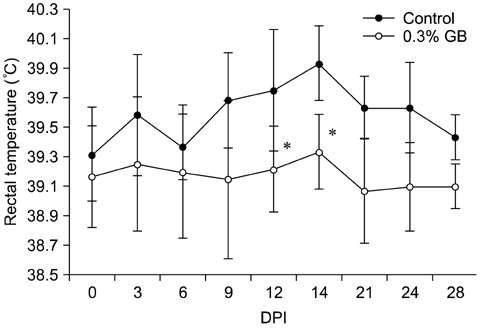
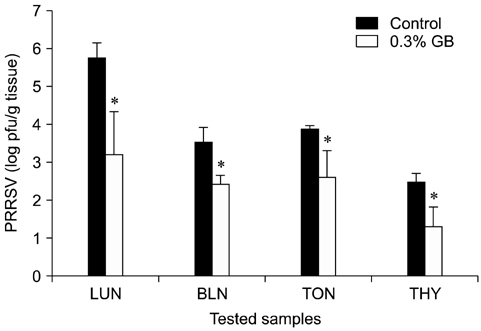
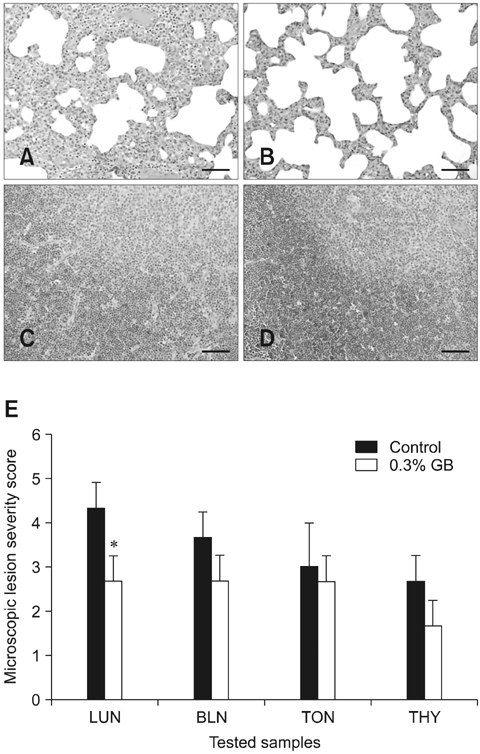
- Germanium biotite (GB) is an aluminosilicate mineral containing 36 ppm germanium. The present study was conducted to better understand the effects of GB on immune responses in a mouse model, and to demonstrate the clearance effects of this mineral against Porcine reproductive and respiratory syndrome virus (PRRSV) in experimentally infected pigs as an initial step towards the development of a feed supplement that would promote immune activity and help prevent diseases. In the mouse model, dietary supplementation with GB enhanced concanavalin A (ConA)-induced lymphocyte proliferation and increased the percentage of CD3+CD8+ T lymphocytes. In pigs experimentally infected with PRRSV, viral titers in lungs and lymphoid tissues from the GB-fed group were significantly decreased compared to those of the control group 12 days post-infection. Corresponding histopathological analyses demonstrated that GB-fed pigs displayed less severe pathological changes associated with PRRSV infection compared to the control group, indicating that GB promotes PRRSV clearance. These antiviral effects in pigs may be related to the ability of GB to increase CD3+CD8+ T lymphocyte production observed in the mice. Hence, this mineral may be an effective feed supplement for increasing immune activity and preventing disease.
MeSH Terms
-
Aluminum Silicates/administration & dosage/*therapeutic use
Animal Feed/analysis
Animals
Antigens, CD3/metabolism
Antigens, CD8/metabolism
Antiviral Agents/administration & dosage/*therapeutic use
Concanavalin A/metabolism
Dietary Supplements/analysis
Disease Models, Animal
Ferrous Compounds/administration & dosage/*therapeutic use
Germanium/administration & dosage/*therapeutic use
Lung/immunology/virology
Lymphocyte Activation/drug effects
Lymphocytes/cytology/drug effects
Lymphoid Tissue/immunology/virology
Mice
Mitogens/metabolism
Porcine Reproductive and Respiratory Syndrome/*drug therapy/pathology/virology
Porcine respiratory and reproductive syndrome virus/*drug effects
Swine
Aluminum Silicates
Antigens, CD3
Antigens, CD8
Antiviral Agents
Concanavalin A
Ferrous Compounds
Germanium
Mitogens
Figure
Cited by 1 articles
-
Dietary germanium biotite supplementation enhances the induction of antibody responses to foot-and-mouth disease virus vaccine in pigs
Jin-A Lee, Bock-Gie Jung, Myunghwan Jung, Tae-Hoon Kim, Han Sang Yoo, Bong-Joo Lee
J Vet Sci. 2014;15(3):443-447. doi: 10.4142/jvs.2014.15.3.443.
Reference
-
1. Aikoh T, Tomokuni A, Matsukii T, Hyodoh F, Ueki H, Otsuki T, Ueki A. Activation-induced cell death in human peripheral blood lymphocytes after stimulation with silicate in vitro. Int J Oncol. 1998; 12:1355–1359.
Article2. Albina E, Carrat C, Charley B. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J Interferon Cytokine Res. 1998; 18:485–490.
Article3. Buddaert W, Van Reeth K, Pensaert M. In vivo and in vitro interferon (IFN) studies with the porcine reproductive and respiratory syndrome virus (PRRSV). Adv Exp Med Biol. 1998; 440:461–467.
Article4. Díaz I, Darwich L, Pappaterra G, Pujols J, Mateu E. Immune responses of pigs after experimental infection with a European strain of porcine reproductive and respiratory syndrome virus. J Gen Virol. 2005; 86:1943–1951.
Article5. Drew TW. A review of evidence for immunosuppression due to porcine reproductive and respiratory syndrome virus. Vet Res. 2000; 31:27–39.
Article6. Hirayama C, Suzuki H, Ito M, Okumura M, Oda T. Propagermanium: a nonspecific immune modulator for chronic hepatitis B. J Gastroenterol. 2003; 38:525–532.
Article7. Jung BG, Ko JH, Cho SJ, Koh HB, Yoon SR, Han DU, Lee BJ. Immune-enhancing effect of fermented Maesil (Prunus mume Siebold & Zucc.) with probiotics against Bordetella bronchiseptica in mice. J Vet Med Sci. 2010; 72:1195–1202.
Article8. Jung BG, Ko JH, Lee BJ. Dietary supplementation with a probiotic fermented four-herb combination enhances immune activity in broiler chicks and increases survivability against Salmonella Gallinarum in experimentally infected broiler chicks. J Vet Med Sci. 2010; 72:1565–1573.
Article9. Jung BG, Toan NT, Cho SJ, Ko JH, Jung YK, Lee BJ. Dietary aluminosilicate supplement enhances immune activity in mice and reinforces clearance of porcine circovirus type 2 in experimentally infected pigs. Vet Microbiol. 2010; 143:117–125.
Article10. Jung K, Renukaradhya GJ, Alekseev KP, Fang Y, Tang Y, Saif LJ. Porcine reproductive and respiratory syndrome virus modifies innate immunity and alters disease outcome in pigs subsequently infected with porcine respiratory coronavirus: implications for respiratory viral co-infections. J Gen Virol. 2009; 90:2713–2723.
Article11. Jung M, Cha SB, Shin SW, Lee WJ, Shin MK, Yoo A, Yoo HS. The effects of Germanium biotite on the adsorptive and inhibition of growth abilities against E. coli and Salmonella spp. in vitro. Korean J Vet Res. 2012; 52:33–38.
Article12. Jung M, Jung BG, Cha SB, Shin MK, Lee WJ, Shin SW, Lee JA, Jung YK, Lee BJ, Yoo HS. The effects of germanium biotite supplement as a prophylactic agent against respiratory infection in calves. Pak Vet J. 2012; 32:319–324.13. Kim HS, Kwang J, Yoon IJ, Joo HS, Frey ML. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch Virol. 1993; 133:477–483.
Article14. Kim WI, Kim JJ, Cha SH, Yoon KJ. Different biological characteristics of wild-type porcine reproductive and respiratory syndrome viruses and vaccine viruses and identification of the corresponding genetic determinants. J Clin Microbiol. 2008; 46:1758–1768.
Article15. Mateu E, Diaz I. The challenge of PRRS immunology. Vet J. 2008; 177:345–351.
Article16. Meier WA, Galeota J, Osorio FA, Husmann RJ, Schnitzlein WM, Zuckermann FA. Gradual development of the interferon-γ response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology. 2003; 309:18–31.
Article17. Moser M, Leo O. Key concepts in immunology. Vaccine. 2010; 28:Suppl 3. C2–C13.
Article18. Opriessnig T, Thacker EL, Yu S, Fenaux M, Meng XJ, Halbur PG. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet Pathol. 2004; 41:624–640.
Article19. Renukaradhya GJ, Alekseev K, Jung K, Fang Y, Saif LJ. Porcine reproductive and respiratory syndrome virus-induced immunosuppression exacerbates the inflammatory response to porcine respiratory coronavirus in pigs. Viral Immunol. 2010; 23:457–466.
Article20. Suradhat S, Thanawongnuwech R. Upregulation of interleukin-10 gene expression in the leukocytes of pigs infected with porcine reproductive and respiratory syndrome virus. J Gen Virol. 2003; 84:2755–2760.
Article21. Tomai M, Kotb M, Majumdar G, Beachey EH. Superantigenicity of streptococcal M protein. J Exp Med. 1990; 172:359–362.
Article22. Ueki A, Yamaguchi M, Ueki H, Watanabe Y, Ohsawa G, Kinugawa K, Kawakami Y, Hyodoh F. Polyclonal human T-cell activation by silicate in vitro. Immunology. 1994; 82:332–335.23. Yoon KJ, Wu LL, Zimmerman JJ, Hill HT, Platt KB. Antibody-dependent enhancement (ADE) of porcine reproductive and respiratory syndrome virus (PRRSV) infection in pigs. Viral Immunol. 1996; 9:51–63.
Article24. Zimmerman J, Benfield DA, Murtaugh MP, Osorio F, Stevenson GW, Tottemorell M. PRRS virus (Porcine Arterivirus). In : Straw BE, Zimmerman JJ, D'Allaire S, Taylor DJ, editors. Diseases of Swine. 9th ed. Ames: Blackwell;2006. p. 387–417.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dietary germanium biotite supplementation enhances the induction of antibody responses to foot-and-mouth disease virus vaccine in pigs
- Pathologic studies in lymph nodes of pigs infected with porcine circovirus type 2, porcine reproductive and respiratory syndrome virus
- Porcine reproductive and respiratory syndrome virus vaccine does not fit in classical vaccinology
- Porcine ear necrosis syndrome by coinfection of porcine reproductive and respiratory syndrome virus and Staphylococcus hyicus
- Evaluation of the efficacy of an attenuated live vaccine based on virulent porcine reproductive and respiratory syndrome virus 2 in young pigs