Korean J Physiol Pharmacol.
2013 Oct;17(5):417-422. 10.4196/kjpp.2013.17.5.417.
Ineffective Doses of Dexmedetomidine Potentiates the Antinociception Induced by Morphine and Fentanyl in Acute Pain Model
- Affiliations
-
- 1Department of Anesthesiology, Cumhuriyet University School of Medicine, Sivas 58140, Turkey. cevdetduger@gmail.com
- 2Department of Pharmacology, Cumhuriyet University School of Medicine, Sivas 58140, Turkey.
- KMID: 1500236
- DOI: http://doi.org/10.4196/kjpp.2013.17.5.417
Abstract
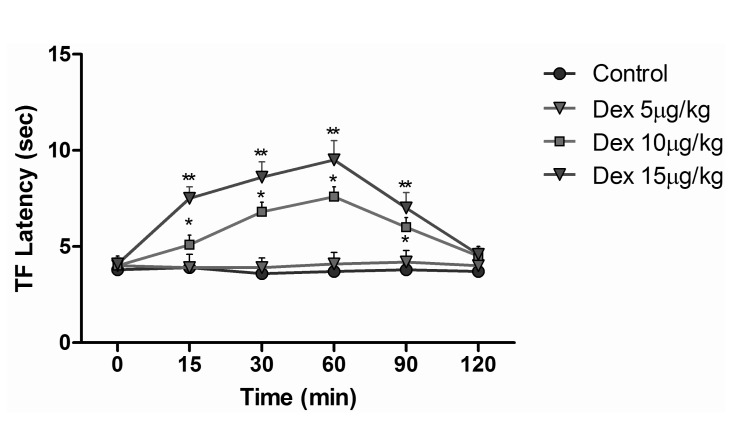
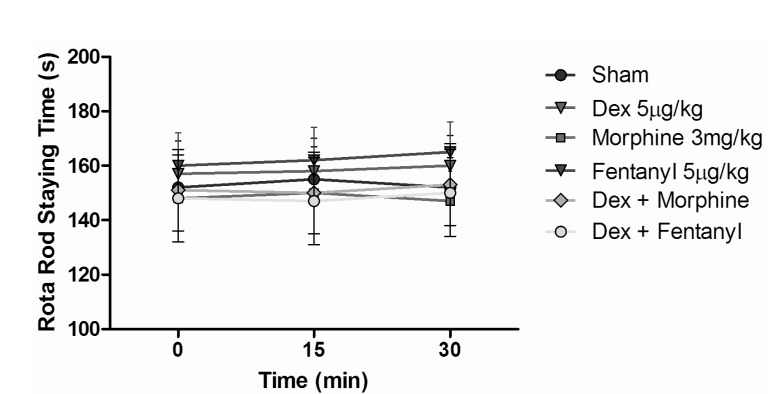
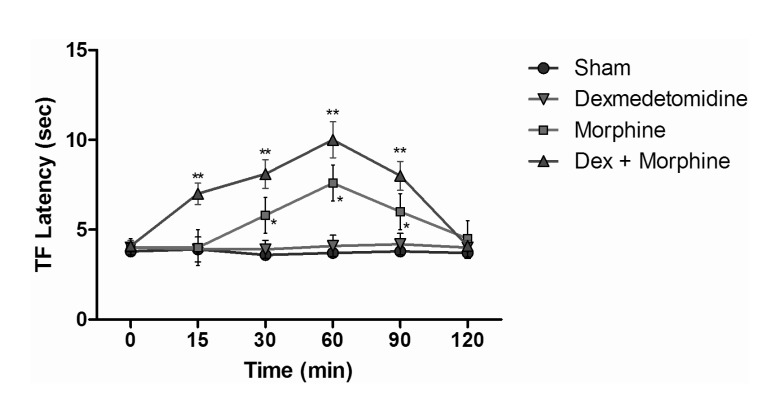
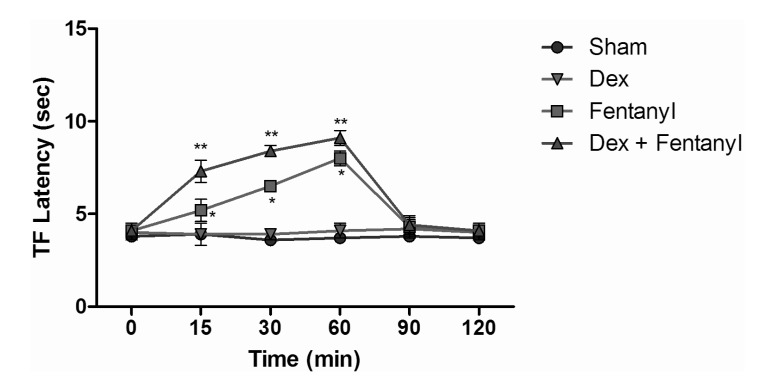
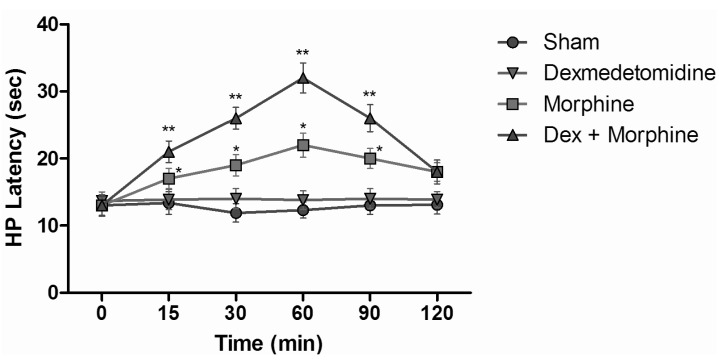
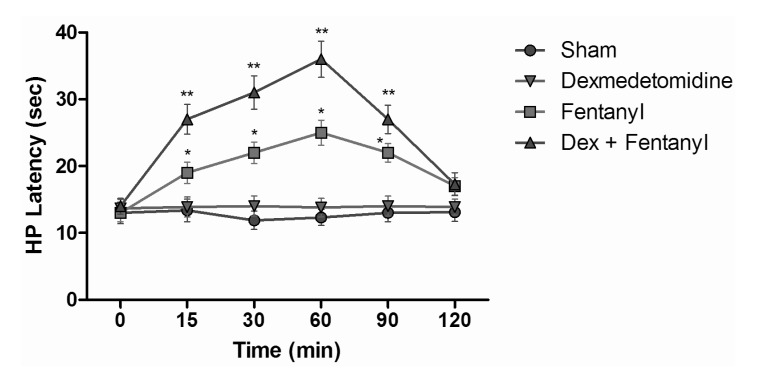
- The aim of this study was to evaluate the synergistic potentiation effect of ineffective doses of dexmedetomidine on antinociception induced by morphine and fentanyl in acute pain model in rats. Seventy albino Wistar rats were separated into 7 groups. Data for the control and sham groups were recorded. The ineffective dose of dexmedetomidine was investigated and found to be 3 micro g/kg. Each group was administered the following medications: 3 mg/kg morphine (intraperitoneal) to Group 3, 5 microg/kg fentanyl (intraperitoneal) to Group 4, dexmedetomidine 3 micro g/kg (subcutaneously) to Group 5, dexmedetomidine 3 microg/kg (subcutaneous)+3 mg/kg morphine (intraperitoneal) to Group 6 and finally 3 microg/kg dexmedetomidine (subcutaneous)+5 microg/kg fentanyl (intraperitoneal) to Group 7. Just before the application and 15, 30, 60, 90 and 120 min after the administration of medication, two measurements of tail flick (TF) and hot plate (HP) tests were performed. The averages of the measurements were recorded. TF and HP latencies were the main outcomes. The analgesic effect of the combinations with dexmedetomidine+morphine (Group 6) and dexmedetomidine+fentanyl (Group 7), compared to the analgesic effect of morphine alone and fentanyl alone was significantly higher at 15, 30, 60 and 90 minutes after administration. In this study, dexmedetomidine in ineffective doses, when combined with morphine and fentanyl, potentiates the effects of both morphine and fentanyl.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Dexmedetomidine Modulates Histamine-induced Ca2+ Signaling and Pro-inflammatory Cytokine Expression
Dongki Yang, Jeong Hee Hong
Korean J Physiol Pharmacol. 2015;19(5):413-420. doi: 10.4196/kjpp.2015.19.5.413.Ferroptosis inhibitor ferrostatin-1 attenuates morphine tolerance development in male rats by inhibiting dorsal root ganglion neuronal ferroptosis
Hasan Dirik, Ahmet Şevki Taşkıran, Ziad Joha
Korean J Pain. 2024;37(3):233-246. doi: 10.3344/kjp.24042.
Reference
-
1. Hernández L, Romero A, Almela P, García-Nogales P, Laorden ML, Puig MM. Tolerance to the antinociceptive effects of peripherally administered opioids. Expression of beta-arrestins. Brain Res. 2009; 1248:31–39. PMID: 19026993.2. Ballesteros-Yanez I, Ambrosio E, Pérez J, Torres I, Miguéns M, García-Lecumberri C, DeFelipe J. Morphine self-administration effects on the structure of cortical pyramidal cells in addiction-resistant rats. Brain Res. 2008; 1230:61–72. PMID: 18657522.
Article3. Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm DH. Effect of berberine on depression- and anxiety-like behaviors and activation of the noradrenergic system induced by development of morphine dependence in rats. Korean J Physiol Pharmacol. 2012; 16:379–386. PMID: 23269899.
Article4. Pergolizzi J, Böger RH, Budd K, Dahan A, Erdine S, Hans G, Kress HG, Langford R, Likar R, Raffa RB, Sacerdote P. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract. 2008; 8:287–313. PMID: 18503626.
Article5. Lanza FL. A guideline for the treatment and prevention of NSAID-induced ulcers. Members of the Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol. 1998; 93:2037–2046. PMID: 9820370.6. Nader ND, Li CM, Dosluoglu HH, Ignatowski TA, Spengler RN. Adjuvant therapy with intrathecal clonidine improves postoperative pain in patients undergoing coronary artery bypass graft. Clin J Pain. 2009; 25:101–106. PMID: 19333153.
Article7. Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: a review of clinical applications. Curr Opin Anaesthesiol. 2008; 21:457–461. PMID: 18660652.
Article9. Ramabadran K, Bansinath M, Turndorf H, Puig MM. The hyperalgesic effect of naloxone is attenuated in streptozotocin-diabetic mice. Psychopharmacology (Berl). 1989; 97:169–174. PMID: 2498924.
Article10. Kanaan SA, Saadé NE, Haddad JJ, Abdelnoor AM, Atweh SF, Jabbur SJ, Safieh-Garabedian B. Endotoxin-induced local inflammation and hyperalgesia in rats and mice: a new model for inflammatory pain. Pain. 1996; 66:373–379. PMID: 8880861.
Article11. Hamurtekin E, Gurun MS. The antinociceptive effects of centrally administered CDP-choline on acute pain models in rats: the involvement of cholinergic system. Brain Res. 2006; 1117:92–100. PMID: 16942753.
Article12. Sullivan AF, Dashwood MR, Dickenson AH. Alpha 2-adrenoceptor modulation of nociception in rat spinal cord: location, effects and interactions with morphine. Eur J Pharmacol. 1987; 138:169–177. PMID: 3040431.13. Coursin DB, Coursin DB, Maccioli GA. Dexmedetomidine. Curr Opin Crit Care. 2001; 7:221–226. PMID: 11571417.
Article14. Paalzow L. Analgesia produced by clonidine in mice and rats. J Pharm Pharmacol. 1974; 26:361–363. PMID: 4152777.
Article15. Dennis SG, Melzack R, Gutman S, Boucher F. Pain modulation by adrenergic agents and morphine as measured by three pain tests. Life Sci. 1980; 26:1247–1259. PMID: 7392798.
Article16. Chan SH, Lai YY. Effects of aging on pain responses and analgesic efficacy of morphine and clonidine in rats. Exp Neurol. 1982; 75:112–119. PMID: 7060671.
Article17. Pertovaara A, Hämäläinen MM, Kauppila T, Mecke E, Carlson S. Dissociation of the alpha 2-adrenergic antinociception from sedation following microinjection of medetomidine into the locus coeruleus in rats. Pain. 1994; 57:207–215. PMID: 7916451.18. Pertovaara A. Antinociception induced by alpha-2-adrenoceptor agonists, with special emphasis on medetomidine studies. Prog Neurobiol. 1993; 40:691–709. PMID: 8097888.
Article19. Sabbe MB, Penning JP, Ozaki GT, Yaksh TL. Spinal and systemic action of the alpha 2 receptor agonist dexmedetomidine in dogs. Antinociception and carbon dioxide response. Anesthesiology. 1994; 80:1057–1072. PMID: 7912480.20. Ossipov MH, Suarez LJ, Spaulding TC. Antinociceptive interactions between alpha 2-adrenergic and opiate agonists at the spinal level in rodents. Anesth Analg. 1989; 68:194–200. PMID: 2537587.21. Meert TF, De Kock M. Potentiation of the analgesic properties of fentanyl-like opioids with alpha 2-adrenoceptor agonists in rats. Anesthesiology. 1994; 81:677–688. PMID: 7916547.22. Slingsby LS, Murrell JC, Taylor PM. Combination of dexmedetomidine with buprenorphine enhances the antinociceptive effect to a thermal stimulus in the cat compared with either agent alone. Vet Anaesth Analg. 2010; 37:162–170. PMID: 20230567.
Article23. Przesmycki K, Dzieciuch JA, Czuczwar SJ, Kleinrok Z. Isobolographic analysis of interaction between intrathecal morphine and clonidine in the formalin test in rats. Eur J Pharmacol. 1997; 337:11–17. PMID: 9389375.
Article24. Puke MJ, Wiesenfeld-Hallin Z. The differential effects of morphine and the alpha 2-adrenoceptor agonists clonidine and dexmedetomidine on the prevention and treatment of experimental neuropathic pain. Anesth Analg. 1993; 77:104–109. PMID: 8100405.25. Guneli E, Karabay Yavasoglu NU, Apaydin S, Uyar M, Uyar M. Analysis of the antinociceptive effect of systemic administration of tramadol and dexmedetomidine combination on rat models of acute and neuropathic pain. Pharmacol Biochem Behav. 2007; 88:9–17. PMID: 17651791.
Article26. Gunes Y, Ozbek TH, Gunduz HM, Gedik YE, Erman T, Ozcengiz D. Patient-controlled analgesia: comparison of morphine to dexmedetomidine plus morphine in patients undergoing laminectomy. Neurosurg Q. 2008; 18:178–181.27. Mantz J. Alpha2-adrenoceptor agonists: analgesia, sedation, anxiolysis, haemodynamics, respiratory function and weaning. Clin Anaesthesiol. 2000; 14:433–448.
Article28. Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000; 59:263–268. PMID: 10730549.
Article29. Aantaa R, Kallio A, Virtanen R. Dexmedetomidine, a novel alpha-2-adrenergic agonist. A review of its pharmacodynamic characteristics. Drugs Future. 1993; 18:49–56.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Comparison of Postoperative Recovery Time and Ventilatory Support Time in the Pediatric Open - heart Patients Anesthetized With Morphine or Fentanyl
- Comparison of Morphine and Fentanyl IV-PCA in Gastrectomy Patient
- The Comparison of Effect of Epidural Morphine and of Morphine-Fentany1-Bupivacaine Mixtere for Analgesia Afrer Cesarean Section
- A comparison of fentanyl and morphine for patient controlled analgesia after laparoscopic cholecystectomy
- Two Cases of Fentanyl Intoxication Through Overusing Fentanyl Patch