J Korean Soc Transplant.
2013 Sep;27(3):114-120. 10.4285/jkstn.2013.27.3.114.
Validation of Tissue Microarrays for the Study of Immunosuppressive Agent-induced Nephrotoxicity
- Affiliations
-
- 1Department of Pathology, Yonsei University College of Medicine, Seoul, Korea. jeong10@yuhs.ac
- 2Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1495868
- DOI: http://doi.org/10.4285/jkstn.2013.27.3.114
Abstract
- BACKGROUND
Tissue microarray analysis (TMA) is a high-throughput method for histologic evaluation, immunohistochemistry, and in situ hybridization using paraffin embedded tissue. Despite its high efficiency as an experimental tool, TMA is limited because it only contains a very small tissue fragment from each case. Therefore, the purpose of this study was to evaluate the validity of TMA in a study of nephrotoxicity caused by immunosuppressants.
METHODS
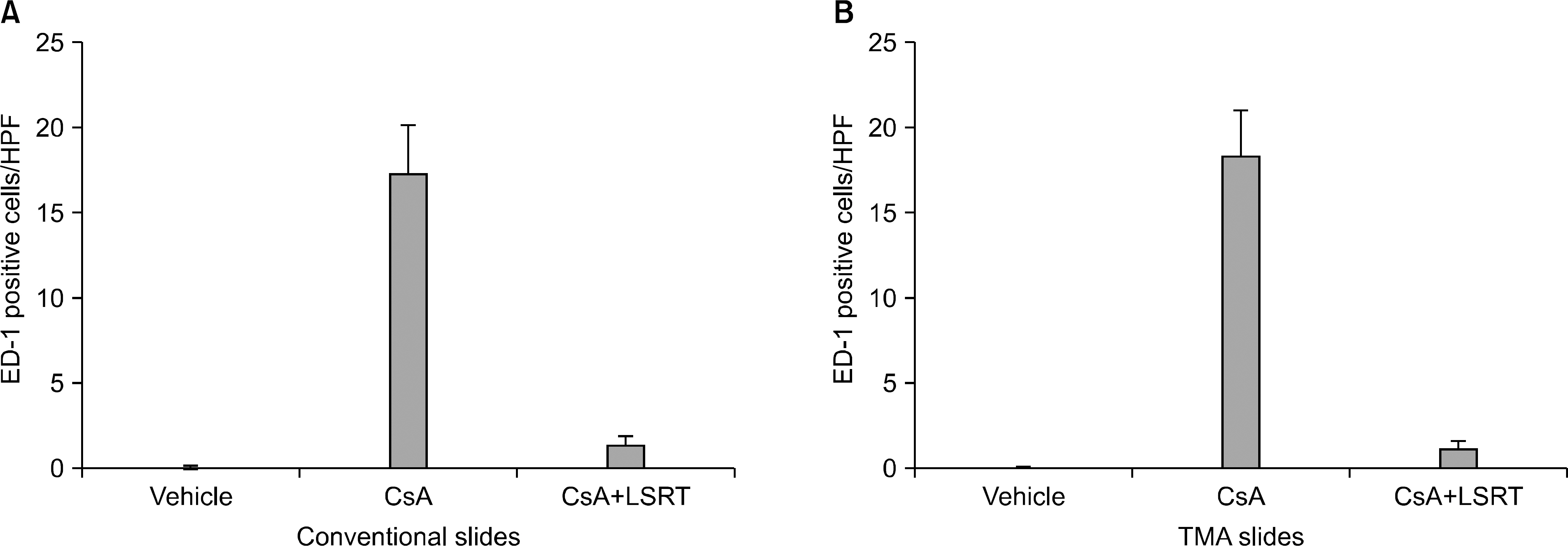
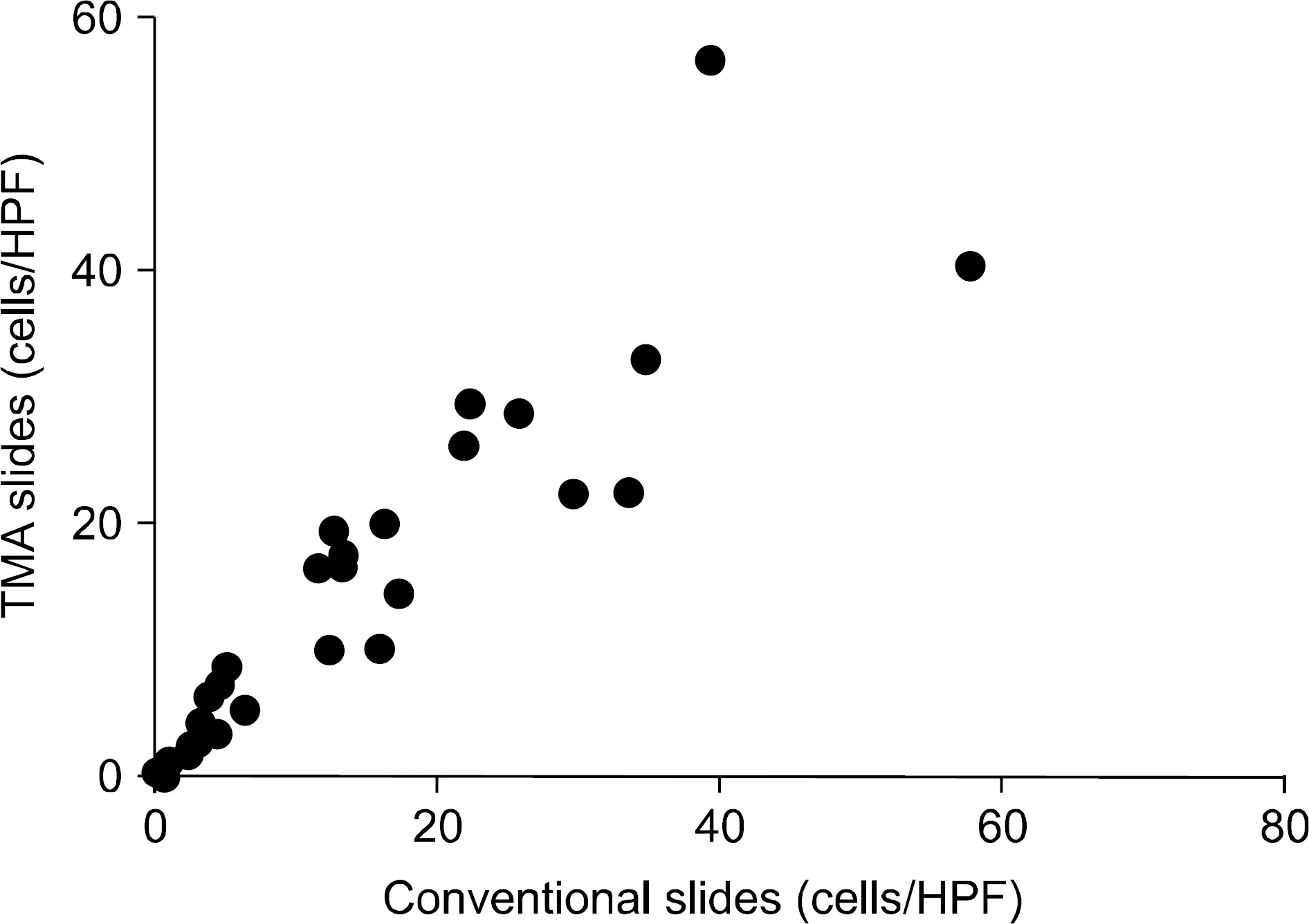
Male Sprague-Dawley rats were treated with vehicle (n=16), cyclosporine (n=23), and cyclosporine plus losartan (n=13) for a maximum of 7 weeks. After animal sacrifice, renal tissues were embedded in paraffin and processed into slides for microscopic examination using conventional methods and the TMA technique. Acute tubular injury, vascular hyaline change, and interstitial fibrosis were scored in both conventional and TMA slides. The number of interstitial macrophages was counted after ED-1 immunohistochemistry and the results also compared between conventional and TMA slides.
RESULTS
The degree of acute tubular injury and interstitial fibrosis showed a significant agreement between conventional and TMA methods (kappa value, 0.79 and 1.00, respectively). The number of interstitial macrophages counted in conventional and TMA slides showed a significant correlation as well (r=0.934, P<0.001). However, the degree of vascular hyaline changes showed less agreement between conventional and TMA methods (kappa value, 0.40).
CONCLUSIONS
TMA is a useful and reliable method for the study of nephrotoxicity induced by immunosuppressive agents. TMA also reflects the findings of conventional methods, especially for acute and chronic tubular and interstitial changes.
MeSH Terms
Figure
Reference
-
1). Camp RL, Neumeister V, Rimm DL. A decade of tissue microarrays: progress in the discovery and validation of cancer biomarkers. J Clin Oncol. 2008; 26:5630–7.
Article2). Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999; 55:713–23.
Article3). Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008; 8:753–60.
Article4). Radhakrishnan R, Solomon M, Satyamoorthy K, Martin LE, Lingen MW. Tissue microarray: a high-throughput molecular analysis in head and neck cancer. J Oral Pathol Med. 2008; 37:166–76.5). Voduc D, Kenney C, Nielsen TO. Tissue microarrays in clinical oncology. Semin Radiat Oncol. 2008; 18:89–97.
Article6). Simon R, Sauter G. Tissue microarrays for miniaturized high-throughput molecular profiling of tumors. Exp He-matol. 2002; 30:1365–72.
Article7). Zhang MZ, Su Y, Yao B, Zheng W, Decaestecker M, Harris RC. Assessing the application of tissue microarray technology to kidney research. J Histochem Cytochem. 2010; 58:413–20.
Article8). Mengel M, Chan S, Climenhaga J, Kushner YB, Regele H, Colvin RB, et al. Banff initiative for quality assurance in transplantation (BIFQUIT): reproducibility of C4d immunohistochemistry in kidney allografts. Am J Transplant. 2013; 13:1235–45.
Article9). Pinder SE, Brown JP, Gillett C, Purdie CA, Speirs V, Thompson AM, et al. The manufacture and assessment of tissue microarrays: suggestions and criteria for analysis, with breast cancer as an example. J Clin Pathol. 2013; 66:169–77.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cisplatin-induced Kidney Dysfunction and Perspectives on Improving Treatment Strategies
- Human kidney organoids model the tacrolimus nephrotoxicity and elucidate the role of autophagy
- Adult Onset Still's Disease Developed in a Patient with Membranous Nephropathy Treated with Immunosuppressive Agent
- Ultrastructural Changes and Expression of Transforming Growth Factor-beta1 in Tacrolimus- Induced Nephropathy
- Chronic Cyclosporine Nephrotoxicity: New Insights and Preventive Strategies