Korean J Physiol Pharmacol.
2012 Oct;16(5):361-365. 10.4196/kjpp.2012.16.5.361.
Gecko Proteins Exert Anti-Tumor Effect against Cervical Cancer Cells Via PI3-Kinase/Akt Pathway
- Affiliations
-
- 1Department of Biological Science, Dong-A University, Busan 604-714, Korea. jwchung@dau.ac.kr
- 2Department of Food and Nutrition, Chonnam National University, Gwangju 500-757, Korea.
- 3Department of Agronomy, Gyeongsang National University, Jinju 660-701, Korea.
- KMID: 1493971
- DOI: http://doi.org/10.4196/kjpp.2012.16.5.361
Abstract
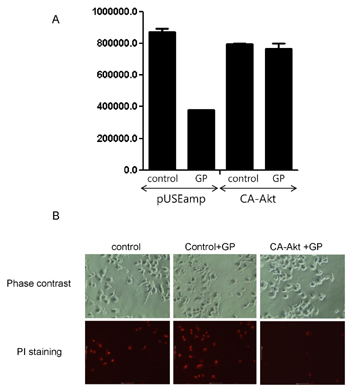
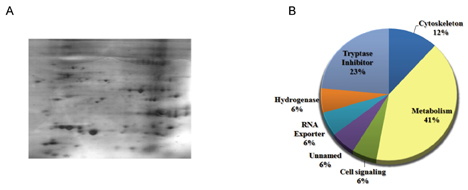
- Anti-tumor activity of the proteins from Gecko (GP) on cervical cancer cells, and its signaling mechanisms were assessed by viable cell counting, propidium iodide (PI) staining, and Western blot analysis. GP induced the cell death of HeLa cells in a dose-dependent manner while it did not affect the viability of normal cells. Western blot analysis showed that GP decreased the activation of Akt, and co-administration of GP and Akt inhibitors synergistically exerted anti-tumor activities on HeLa cells, suggesting the involvement of PI3-kinase/Akt pathway in GP-induced cell death of the cancer cells. Indeed, the cytotoxic effect of GP against HeLa cells was inhibited by overexpression of constituvely active form of Akt in HeLa cells. The candidates of the functional proteins in GP were analyzed by Mass-spectrum. Taken together, our results suggest that GP elicits anti-tumor activity against HeLa cells by inhibition of PI3-kinase/Akt pathway.
Keyword
MeSH Terms
Figure
Reference
-
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010. 127:2893–2917.2. Liu F, Wang JG, Wang SY, Li Y, Wu YP, Xi SM. Antitumor effect and mechanism of Gecko on human esophageal carcinoma cell lines in vitro and xenografted sarcoma 180 in Kunming mice. World J Gastroenterol. 2008. 14:3990–3996.3. Yang JX, Wang XM. Progress study and research on treating tumor of Gecko. Shijie Huaren Xiaohua Zazhi. 2006. 14:2428–2431.4. Wu BD. Treatment of 105 cases of esophagus tumor by compound recipe of Gecko. Zhongguo Zhongxiyi Jiehe Zazhi. 1999. 19:502.5. Song P, Wang XM, Xie S. Experimental study on mechanisms of lyophilized powder of fresh gekko Chinenis in inhibiting H22 hepatocarcinoma angiogenesis. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2006. 26:58–62.6. Wang SJ, Zhang XY, Lu LB. Research and application of the pharmacological testing method using serum with Chinese herbal medicine. Zhongguo Shouyao Zazhi. 2004. 38:35–37.7. Xue J, Xie ML. Advances in studies on methodology in serum pharmacology of Chinese materia medica. Zhongcaoyao. 2003. 34:9–11.8. Iwama H, Amagaya S, Ogihara Y. Effect of shosaikoto, a Japanese and Chinese traditional herbal medicinal mixture, on the mitogenic activity of lipopolysaccharide: a new pharmacological testing method. J Ethnopharmacol. 1987. 21:45–53.9. Ferreira FS, Brito SV, Saraiva RA, Araruna MK, Menezes IR, Costa JG, Coutinho HD, Almeida WO, Alves RR. Topical anti-inflammatory activity of body fat from the lizard Tupinambis merianae. J Ethnopharmacol. 2010. 130:514–520.10. Chen D, Zhang X, Du Y, Jia B, Ka W, Sun D, Yao W, Wen Z. Effects of Gekko sulfated polysaccharide-protein complex on the defective biorheological characters of dendritic cells under tumor microenvironment. Cell Biochem Biophys. 2012. 62:193–201.11. Wu XZ, Chen D, Han XQ. Anti-migration effects of Gekko sulfated glycopeptide on human hepatoma SMMC-7721 cells. Molecules. 2011. 16:4958–4970.12. Chen D, Yao WJ, Zhang XL, Han XQ, Qu XY, Ka WB, Sun DG, Wu XZ, Wen ZY. Effects of Gekko sulfated polysaccharide-protein complex on human hepatoma SMMC-7721 cells: inhibition of proliferation and migration. J Ethnopharmacol. 2010. 127:702–708.13. Zhu YF, Yu BH, Li DL, Ke HL, Guo XZ, Xiao XY. PI3K expression and PIK3CA mutations are related to colorectal cancer metastases. World J Gastroenterol. 2012. 18:3745–3751.14. Hussain AR, Ahmed SO, Ahmed M, Khan OS, Al Abdulmohsen S, Platanias LC, Al-Kuraya KS, Uddin S. Cross-talk between NFkB and the PI3-kinase/AKT pathway can be targeted in primary effusion lymphoma (PEL) cell lines for efficient apoptosis. PLoS One. 2012. 7:e39945.15. Qian C, Lai CJ, Bao R, Wang DG, Wang J, Xu GX, Atoyan R, Qu H, Yin L, Samson M, Zifcak B, Ma AW, Dellarocca S, Borek M, Zhai HX, Cai X, Voi M. Cancer network disruption by a single molecule inhibitor targeting both histone deacetylase activity and phosphatidylinositol 3-kinase signaling. Clin Cancer Res. 2012. 18:4104–4113.16. Coso S, Zeng Y, Opeskin K, Williams ED. Vascular endothelial growth factor receptor-3 directly interacts with phosphatidylinositol 3-kinase to regulate lymphangiogenesis. PLoS One. 2012. 7:e39558.17. Hong DS, Bowles DW, Falchook GS, Messersmith WA, George GC, O'Bryant CL, Vo AC, Klucher K, Herbst RS, Eckhardt SG, Peterson S, Hausman DF, Kurzrock R, Jimeno A. A Multicenter Phase I Trial of PX-866, an Oral Irreversible Phosphatidylinositol 3-Kinase Inhibitor, in Patients with Advanced Solid Tumors. Clin Cancer Res. 2012. 18:4173–4182.18. Le TK, Jeong JJ, Kim DH. Clionosterol and ethyl cholestan-22-enol isolated from the rhizome of Polygala tenuifolia inhibit phosphatidylinositol 3-kinase/Akt pathway. Biol Pharm Bull. 2012. 35:1379–1383.19. Clemente A, Carmen Marín-Manzano M, Jiménez E, Carmen Arques M, Domoney C. The anti-proliferative effect of TI1B, a major Bowman-Birk isoinhibitor from pea (Pisum sativum L.), on HT29 colon cancer cells is mediated through protease inhibition. Br J Nutr. 2012. 108:suppl 1. S135–S144.20. Brandi G, Tavolari S, De Rosa F, Di Girolamo S, Agostini V, Barbera MA, Frega G, Biasco G. Antitumoral efficacy of the protease inhibitor gabexate mesilate in colon cancer cells harbouring KRAS, BRAF and PIK3CA mutations. PLoS One. 2012. 7:e41347.21. Sun L, Niu L, Zhu X, Hao J, Wang P, Wang H. Antitumour effects of a protease inhibitor, nelfinavir, in hepatocellular carcinoma cancer cells. J Chemother. 2012. 24:161–166.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory effect of capsaicin on B16-F10 melanoma cell migration via the phosphatidylinositol 3-kinase/Akt/Rac1 signal pathway
- Insulin-Like Growth Factor 1 Actions in Developing Brain and the Interaction with Wnt Pathway
- Comparative effects of PKB- alpha and PKC- zeta on the phosphorylation of GLUT4-containing vesicles in rat adipocytes
- Anti-proliferative Effect of 15,16-Dihydrotanshinone I Through Cell Cycle Arrest and the Regulation of AMP-activated Protein Kinase/Akt/mTOR and Mitogen-activated Protein Kinase Signaling Pathway in Human Hepatocellular Carcinoma Cells
- The Tumor Suppressor Function of PTEN/MMAC1 through the Regulation of IGFs and IGFBPs