Korean J Physiol Pharmacol.
2008 Oct;12(5):281-286. 10.4196/kjpp.2008.12.5.281.
Tyrphostin ErbB2 Inhibitors AG825 and AG879 Have Non-specific Suppressive Effects on gp130/ STAT3 Signaling
- Affiliations
-
- 1Department of Physiology, Medical Science Research Institute, College of Medicine, Dong-A University, Busan, Korea. phwantae@dau.ac.kr
- 2Department of Microbiology, Medical Science Research Institute, College of Medicine, Dong-A University, Busan, Korea.
- 3Department of Biochemistry and Molecular Biology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1486111
- DOI: http://doi.org/10.4196/kjpp.2008.12.5.281
Abstract
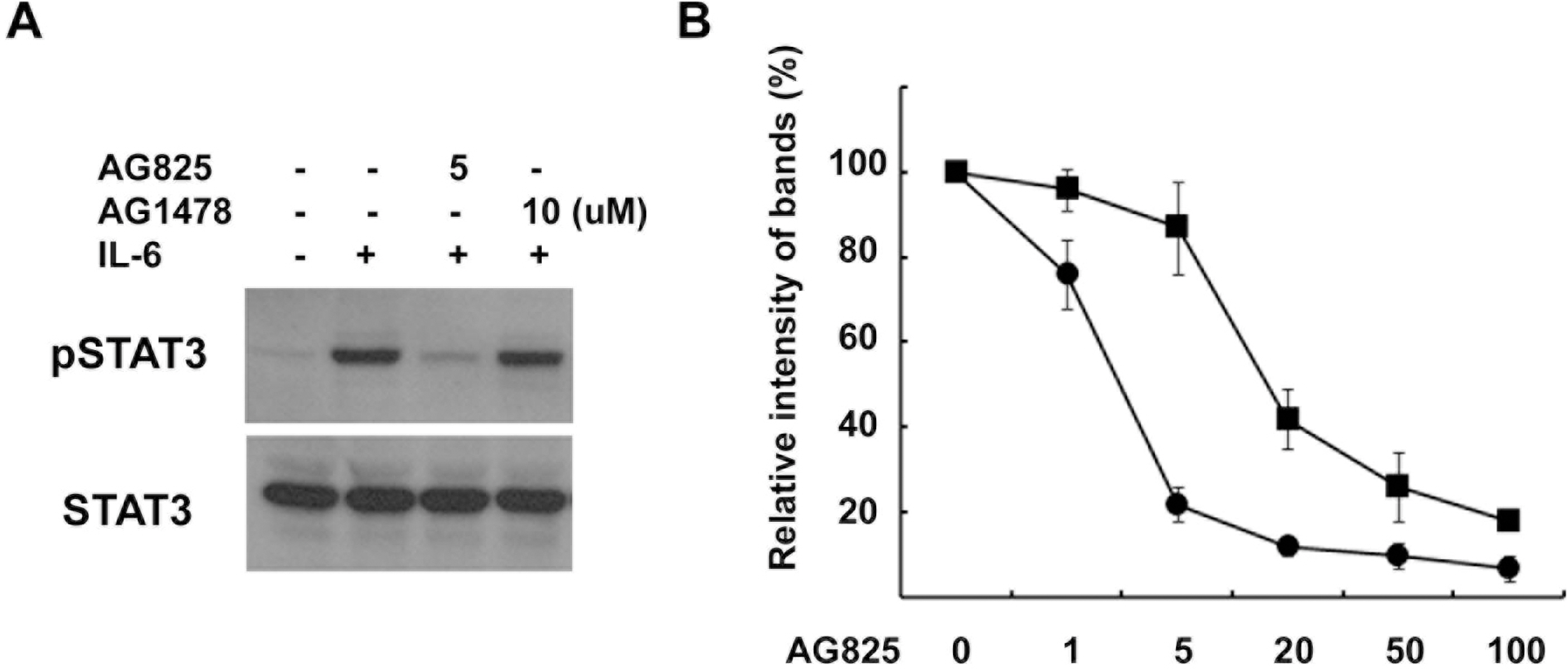
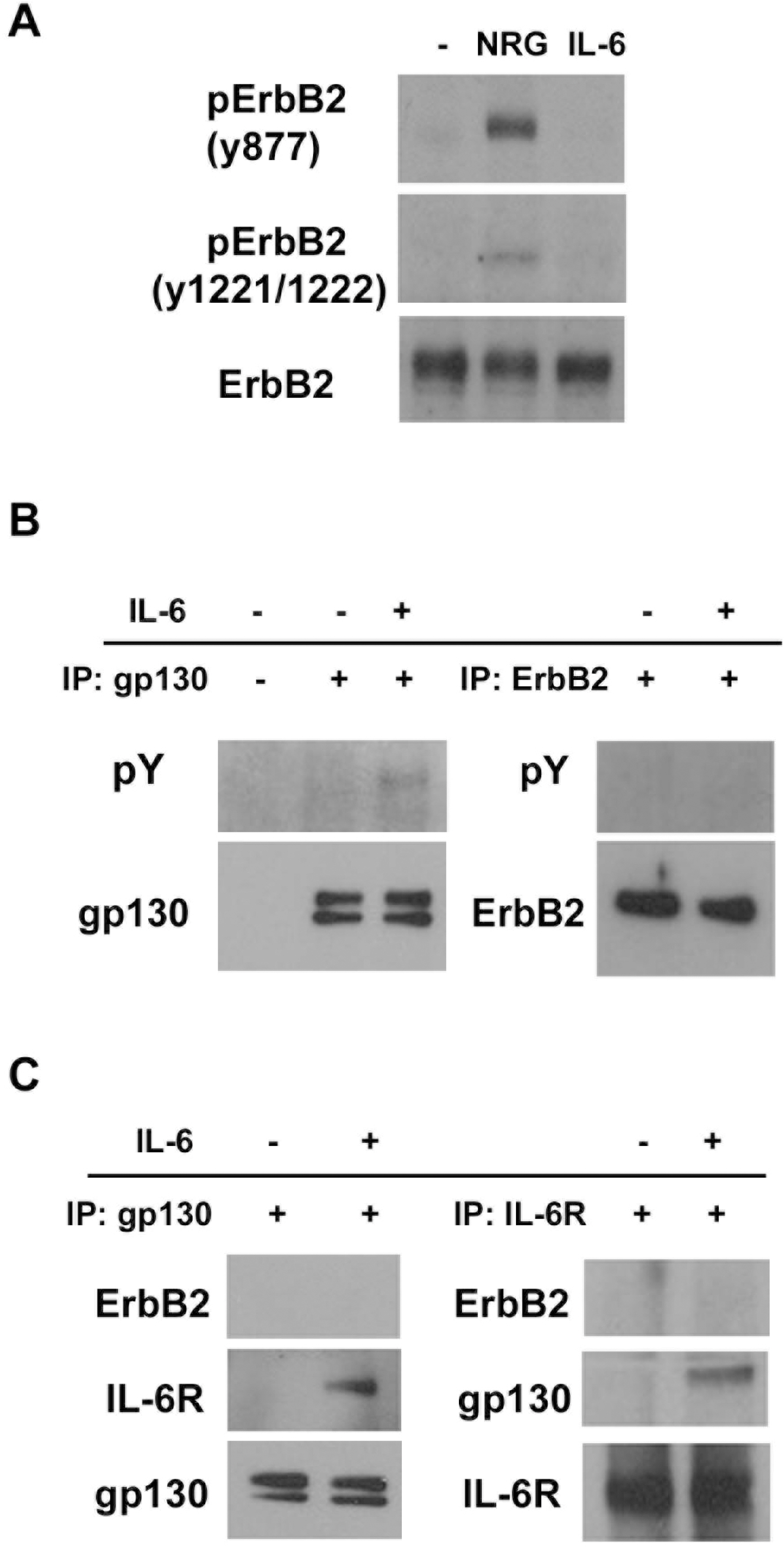
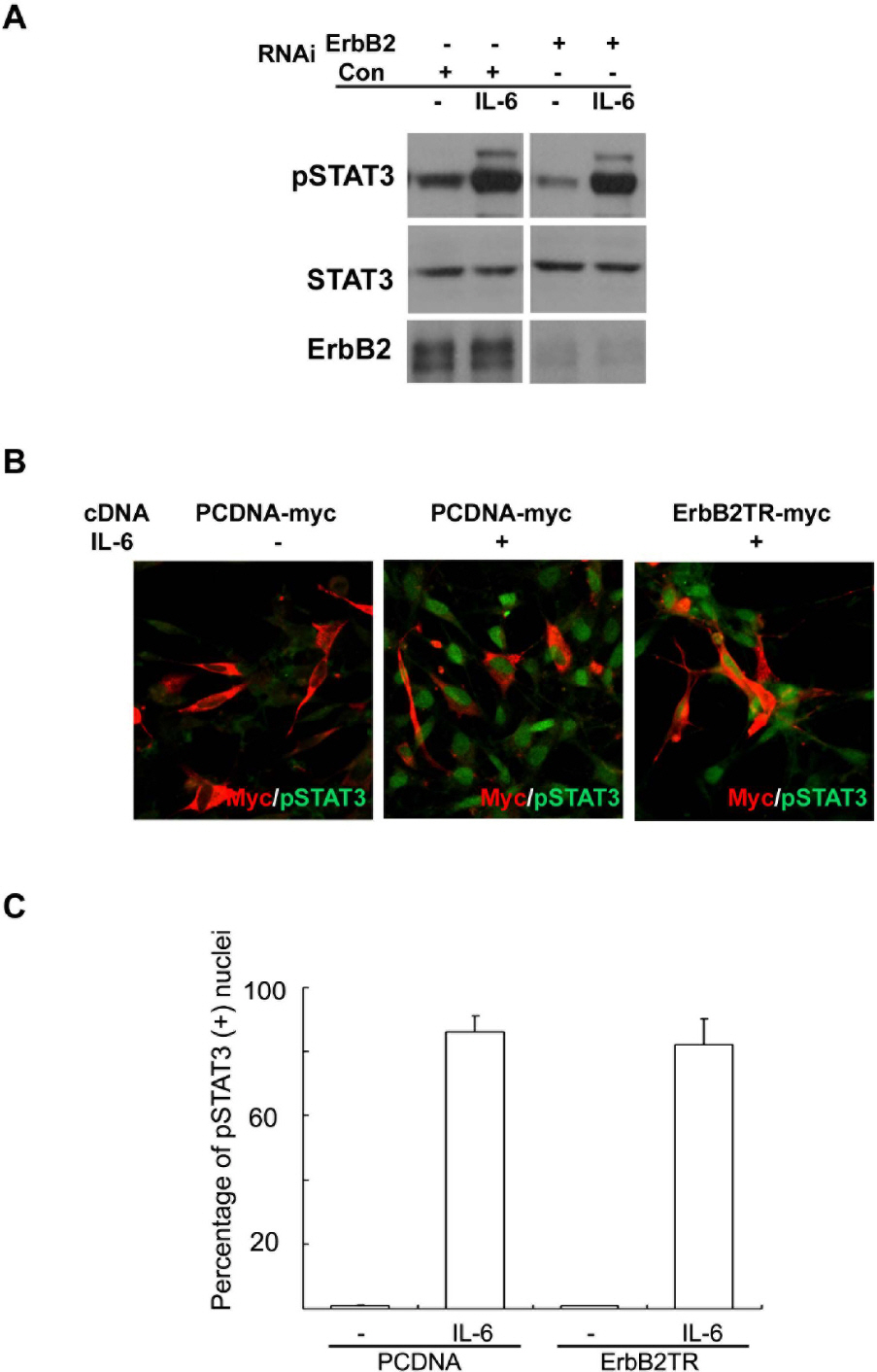
- Although the interaction between gp130 and the ErbB family has frequently been shown in cancer cells, the mechanism of this interaction remains unclear and controversial. In the present study, we found that specific tyrphostin inhibitors of ErbB2 (AG825 and AG879), but not ErbB1 inhibitor (AG1478), suppressed IL-6-induced tyrosine phosphorylation of STAT3 in schwannoma cells. However, biochemical evidence for transactivation of ErbB2 by IL-6 was not observed. Additionally, the inhibition of ErbB2 expression, with either a specific RNAi or transfection of an ErbB2 mutant lacking the intracellular domain did not inhibit the IL-6-induced tyrosine phosphorylation of STAT3. Thus, it seems that tyrphostins, which are known as specific inhibitors of the ErbB2 kinase, may have non-specific suppressive effects on the IL-6/STAT3 pathway.
Keyword
MeSH Terms
Figure
Reference
-
Badache A., Hynes NE. Interleukin 6 inhibits proliferation and, in cooperation with an epidermal growth factor receptor autocrine loop, increases migration of T47D breast cancer cells. Cancer Res. 61:383–391. 2001.Battle TE., Frank DA. The role of STATs in apoptosis. Curr Mol Med. 2:381–392. 2002.
ArticleCarroll SL., Miller ML., Frohnert PW., Kim SS., Corbett JA. Expression of neuregulins and their putative receptors, ErbB2 and ErbB3, is induced during Wallerian degeneration. J Neurosci. 17:1642–1659. 1997.
ArticleChan KS., Sano S., Kiguchi K., Anders J., Komazawa N., Takeda J., DiGiovanni J. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J Clin Invest. 114:720–728. 2004.
ArticleChen ZL., Yu WM., Strickland S. Peripheral regeneration. Annu Rev Neurosci. 30:209–233. 2007.
ArticleCheng L., Esch FS., Marchionni MA., Mudge AW. Control of Schwann cell survival and proliferation: autocrine factors and neuregulins. Mol Cell Neurosci. 12:141–156. 1998.
ArticleChoi BH., Choi JS., Rhie DJ., Yoon SH., Min DS., Jo YH., Kim MS., Hahn SJ. Direct inhibition of the cloned Kv1.5 channel by AG-1478, a tyrosine kinase inhibitor. Am J Physiol Cell Physiol. 282:1461–1468. 2002.
ArticleGazit A., Osherov N., Posner I., Yaish P., Poradosu E., Gilon C., Levitzki A. Tyrphostins. 2. Heterocyclic and alpha-substituted benzylidenemalononitrile tyrphostins as potent inhibitors of EGF receptor and ErbB2/neu tyrosine kinases. J Med Chem. 34:1896–1907. 1991.Grant SL., Hammacher A., Douglas AM., Goss GA., Mansfield RK., Heath JK., Begley CG. An unexpected biochemical and functional interaction between gp130 and the EGF receptor family in breast cancer cells. Oncogene. 21:460–474. 2002.
ArticleGrunt TW., Tomek K., Wagner R., Puckmair K., Kainz B., Rünzler D., Gaiger A., Köhler G., Zielinski CC. Upregulation of retinoic acid receptor-beta by the epidermal growth factor-receptor inhibitor PD153035 is not mediated by blockade of ErbB pathways. J Cell Physiol. 211:803–815. 2007.Huijbregts RP., Roth KA., Schmidt RE., Carroll SL. Hypertrophic neuropathies and malignant peripheral nerve sheath tumors in transgenic mice overexpressing glial growth factor beta3 in myelinating Schwann cells. J Neurosci. 23:7269–7280. 2003.Hynes NE., Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 5:341–354. 2005.
ArticleIwamaru A., Szymanski S., Iwado E., Aoki H., Yokoyama T., Fokt I., Hess K., Conrad C., Madden T., Sawaya R., Kondo S., Priebe W., Kondo Y. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene. 26:2435–2444. 2007.
ArticleKamimura D., Ishihara K., Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 149:1–38. 2003.
ArticleLee HK., Seo IA., Park HK., Park HT. Identification of the basement membrane protein nidogen as a candidate ligand for tumor endothelial marker 7 in vitro and in vivo. FEBS Lett. 580:2253–2257. 2006.
ArticleLee HK., Seo IA., Park HK., Park YM., Ahn KJ., Yoo YH., Park HT. Nidogen is a prosurvival and promigratory factor for adult Schwann cells. J Neurochem. 102:686–698. 2007.
ArticleLee HK., Seo IA., Seo E., Seo SY., Lee HJ., Park HT. Netrin-1 induces proliferation of Schwann cells through Unc5b receptor. Biochem Biophys Res Commun. 362:1057–1062. 2007.
ArticleNg YP. Cheung ZH, Ip NY. STAT3 as a downstream mediator of Trk signaling and functions. J Biol Chem. 281:15636–15644. 2006.Osherov N., Gazit A., Gilon C., Levitzki A. Selective inhibition of the epidermal growth factor and HER2/neu receptors by tyrphostins. J Biol Chem. 268:11134–11142. 1993.
ArticleQiu Y., Ravi L., Kung HJ. Requirement of ErbB2 for signalling by interleukin-6 in prostate carcinoma cells. Nature. 393:83–85. 1998.
ArticleRahaman SO., Harbor PC., Chernova O., Barnett GH., Vogelbaum MA., Haque SJ. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multi-forme cells. Oncogene. 21:8404–8413. 2002.
ArticleSelander KS., Li L., Watson L., Merrell M., Dahmen H., Heinrich PC., Müller-Newen G., Harris KW. Inhibition of gp130 signaling in breast cancer blocks constitutive activation of Stat3 and inhibits in vivo malignancy. Cancer Res. 64:6924–6933. 2004.
ArticleStonecypher MS., Byer SJ., Grizzle WE., Carroll SL. Activation of the neuregulin-1/ErbB signaling pathway promotes the proliferation of neoplastic Schwann cells in human malignant peripheral nerve sheath tumors. Oncogene. 24:5589–5605. 2005.
ArticleTsai CM., Levitzki A., Wu LH., Chang KT., Cheng CC., Gazit A., Perng RP. Enhancement of chemosensitivity by tyrphostin AG825 in high-p185 (neu) expressing non-small cell lung cancer cells. Cancer Res. 56:1068–1074. 1996.Xie Y., Tisi MA., Yeo TT., Longo FM. Nerve growth factor (NGF) loop 4 dimeric mimetics activate ERK and AKT and promote NGF-like neurotrophic effects. J Biol Chem. 275:29868–29874. 2000.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Janus Kinase 2 Inhibitor AG490 Inhibits the STAT3 Signaling Pathway by Suppressing Protein Translation of gp130
- The role of local IL6/JAK2/STAT3 signaling in high glucose–induced podocyte hypertrophy
- Differential Signaling and Virus Production in Calu-3 Cells and Vero Cells upon SARS-CoV-2 Infection
- gp130 is important for the normal morphogenesis of Meckel's cartilage and subsequent mandibular development
- Benzoxathiol derivative BOT-4-one suppresses L540 lymphoma cell survival and proliferation via inhibition of JAK3/STAT3 signaling