Clin Exp Otorhinolaryngol.
2008 Jun;1(2):86-91. 10.3342/ceo.2008.1.2.86.
Establishment and Characterization of an In Vitro Model for Cholesteatoma
- Affiliations
-
- 1Department of Otorhinolaryngology, University Hospital Tzaritza Joanna, Medical University - Sofia, Sofia, Bulgaria.
- 2Department of Otolaryngology, Ajou University School of Medicine, Suwon, Korea. parkkh@ajou.ac.kr
- KMID: 1486059
- DOI: http://doi.org/10.3342/ceo.2008.1.2.86
Abstract
OBJECTIVES
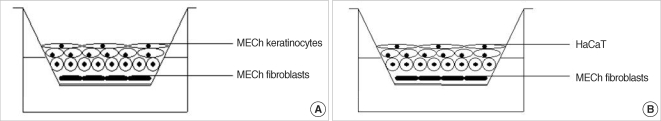
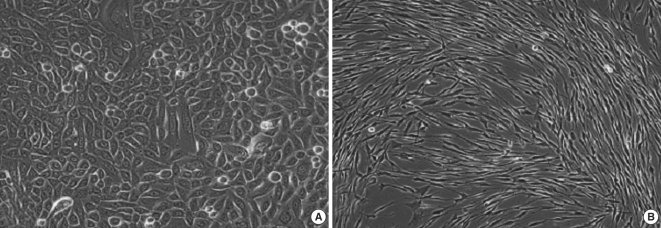
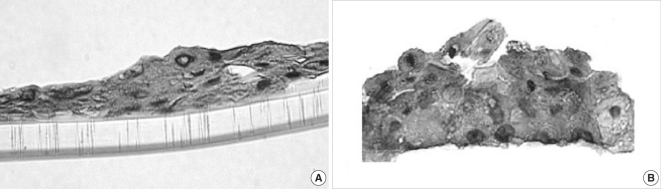
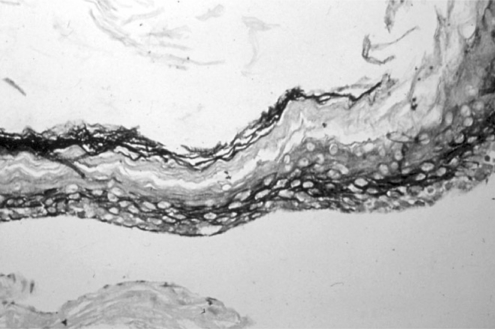
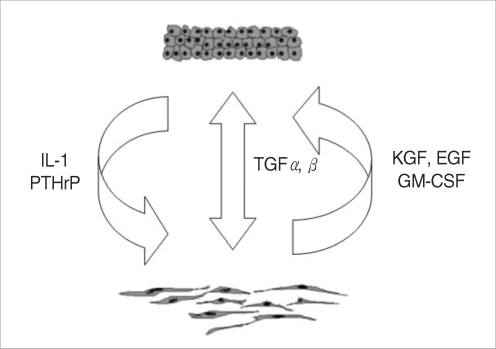
Experimental models are of importance to study the pathogenesis of middle ear cholesteatoma, however, they were not established until now. We aimed to develop in vitro model of middle ear cholesteatoma using primary keratinocytes and fibroblasts isolated from cholesteatoma tissue. HaCaT cell line was used as a "skin equivalent" and to compare the grade of homogeneity between cholesteatoma keratinocytes and HaCaT cells. METHODS: Primary keratinocytes were isolated from cholesteatoma tissue, co-cultured with preliminary prepared feeder layer from cholesteatoma fibroblasts and subsequently air-exposed. The protein profile of cholesteatoma keratinocytes and HaCaT cells was evaluated by means of immunoblot using monoclonal antibody against cytokeratin (CK) 13 and 16. Tissue localization of CK 13 and 16 was accomplished with immunohistochemistry. RESULTS: Different protein profile and stronger expression of CK 13 and 16 were demonstrated in cholesteatoma keratinocytes in comparison with HaCaT cells. Bigger stratification was observed in the 3D-in vitro systems when both cholesteatoma keratinocytes and HaCaT cells were respectively co-cultured with fibroblasts in comparison with the corresponding control groups without fibroblasts. CONCLUSION: 3D-model demonstrates the significance of intercellular interaction between components of cholesteatoma tissue.
Keyword
MeSH Terms
Figure
Reference
-
1. Gray JD. The chronic ear: the treatment of cholesteatoma in children. Proc R Soc Med. 1964; 9. 57:769–771. PMID: 14208009.2. Sadé J, Babiacki A, Pinkus G. The metaplastic and congenital origin of cholesteatoma. Acta Otolaryngol. 1983; Jul–Aug. 96(1-2):119–129. PMID: 6193677.
Article3. Vassalli L, Harris DM, Gradini R, Applebaum EL. Propylene glycol-induced cholesteatoma in chinchilla middle ears. Am J Otolaryngol. 1988; Jul–Aug. 9(4):180–188. PMID: 3228176.
Article4. McGinn MD, Chole RA, Henry KR. Cholesteatoma. Experimental induction in the Mongolian Gerbil, Meriones Unguiculaus. Acta Otolaryngol. 1982; Jan–Feb. 93(1-2):61–67. PMID: 7064697.
Article5. Park K, Chun YM, Kim SM, Lee DH. Experimental cholesteatoma by transplanting a free skin graft in the bulla of Mongolian gerbil. Korean J Otolaryngol - Head Neck Surg. 1996; 7. 39(7):1138–1143.6. Proops DW, Parkinson EK. Tissue culture of human cholesteatomatous keratinocytes. Clin Otolaryngol Allied Sci. 1983; 6. 8(3):165–170. PMID: 6192952.
Article7. Cheshire IM, Blight A, Proops DW. An in vitro growth study on cholesteatoma and normal skin. Clin Otolaryngol Allied Sci. 1995; 10. 20(5):453–460. PMID: 8582080.
Article8. Albers-op t' Hof BM, Peek FA, Huisman MA, Grote JJ. Air-exposed tissue culture of human middle ear epithelium and meatal epidermis: a method to study the advancing front of cholesteatoma. Acta Otolaryngol. 2002; 10. 122(7):720–725. PMID: 12484648.9. Park K, Park HJ, Chun YM. Lim DJ, Casselbrant M, Klein JO, Ogra PL, editors. Immunohistochemical study of cytokeratin expression in experimental cholesteatoma. Recent advances in otitis media, Sixth International Symposium. 1996. Hamilton: B.C. Decker Inc.;p. 275–277.10. Tanaka Y, Kojima H, Miyazaki H, Koga T, Moriyama H. Roles of cytokines and cell cycle regulating substances in proliferation of cholesteatoma epithelium. Laryngoscope. 1999; 7. 109(7 Pt 1):1102–1107. PMID: 10401849.
Article11. Huang T, Yan SD, Huang CC. Colony-stimulating factor in middle ear cholesteatoma. Am J Otolaryngol. 1989; Nov–Dec. 10(6):393–398. PMID: 2688444.
Article12. Cheshire IM, Blight A, Ratcliffe WA, Proops DW, Heath DA. Production of parathyroid-hormone-related protein by cholesteatoma cells in culture. Lancet. 1991; 10. 338(8774):1041–1043. PMID: 1681357.
Article13. Schulz P, Bujía J, Holly A, Shilling V, Kastenbauer E. Possible autocrine growth stimulation of cholesteatoma epithelium by transforming growth factor alpha. Am J Otolaryngol. 1993; Mar–Apr. 14(2):82–87. PMID: 8484481.
Article14. Ergün S, Zheng X, Carlsöö B. Expression of transforming growth factor-alpha and epidermal growth factor receptor in middle ear cholesteatoma. Am J Otol. 1996; 5. 17(3):393–396. PMID: 8817015.15. Lang S, Schilling V, Wollenberg B, Mack B, Nerlich A. Localization of transforming growth factor-beta-expressing cells and comparison with major extracellular components in aural cholesteatoma. Ann Otol Rhinol Laryngol. 1997; 8. 106(8):669–673. PMID: 9270431.16. Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988; 3. 106(3):761–771. PMID: 2450098.
Article17. Boelsma E, Verhoeven MC, Ponec M. Reconstruction of a human skin equivalent using a spontaneously transformed keratinocyte cell line (HaCaT). J Invest Dermatol. 1999; 4. 112(4):489–498. PMID: 10201534.
Article18. Choufani G, Mahillon V, Decaestecker C, Lequeux T, Danguy A, Salmon I, et al. Determination of the levels of expression of sarcolectin and calcyclin and of the percentages of apoptotic but not proliferating cells to enable distinction between recurrent and nonrecurrent cholesteatomas. Laryngoscope. 1999; 11. 109(11):1825–1831. PMID: 10569415.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Retroauricular Cholesteatoma in Soft Tissue after Tympanomastoidectomy
- Development of Animal Models of Otitis Media
- Significance of Langerhans' cells in middle ear cholesteatoma
- Immunohistochemical Study of Thrombomodulin in Experimental Cholesteatoma
- Distribution of Cytokeratins in Cholesteatomas according to the Induction Period of Cholesteatoma in Gerbil