J Bacteriol Virol.
2008 Dec;38(4):221-226. 10.4167/jbv.2008.38.4.221.
Rapid Identification of Rickettsiae using the Real-Time PCR
- Affiliations
-
- 1Department of Microbiology, College of Medicine, Konkuk University, Chugju, Chungchungbuk-Do, Korea. shlee@kku.ac.kr
- 2Department of Microbiology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1483962
- DOI: http://doi.org/10.4167/jbv.2008.38.4.221
Abstract
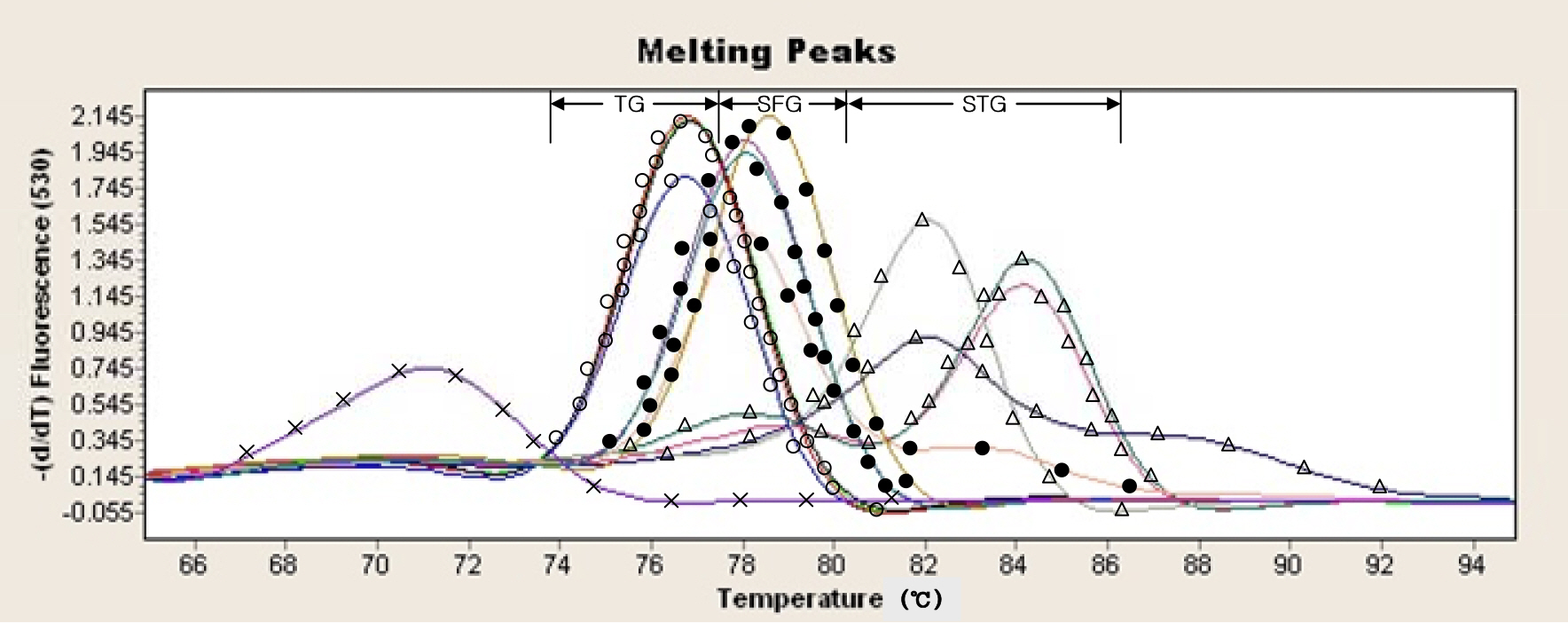
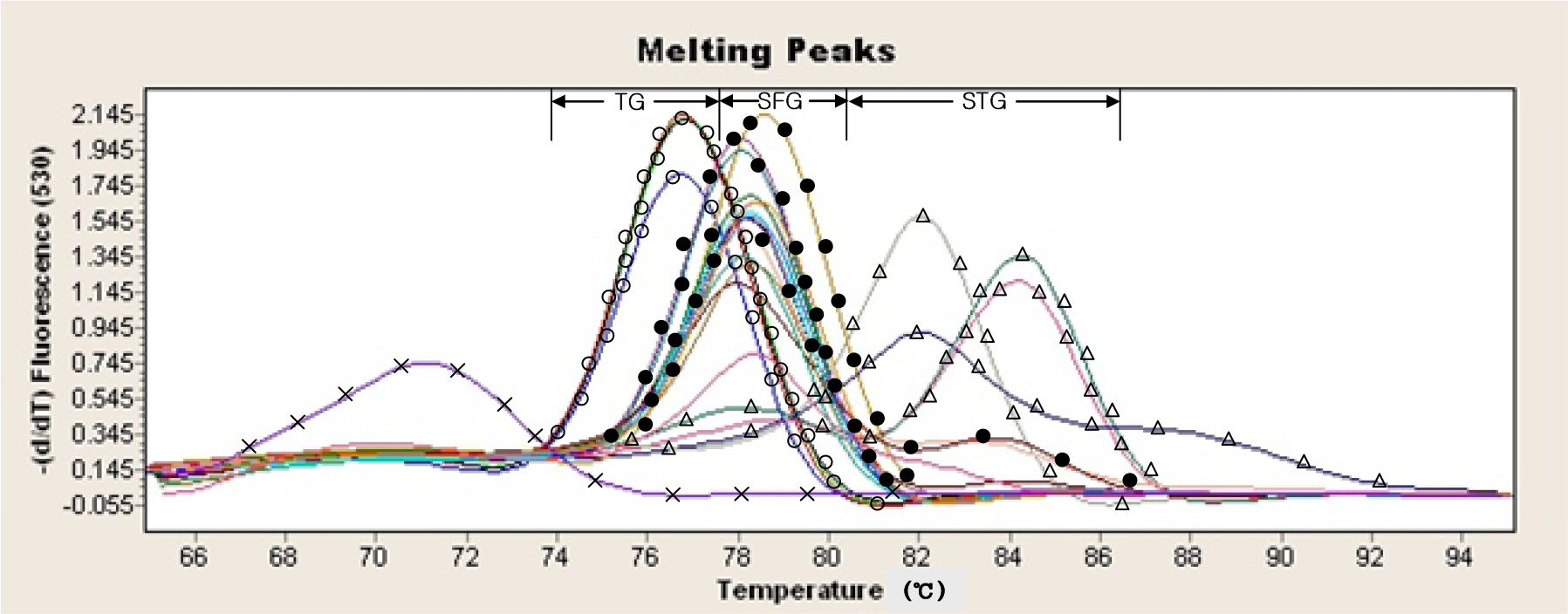
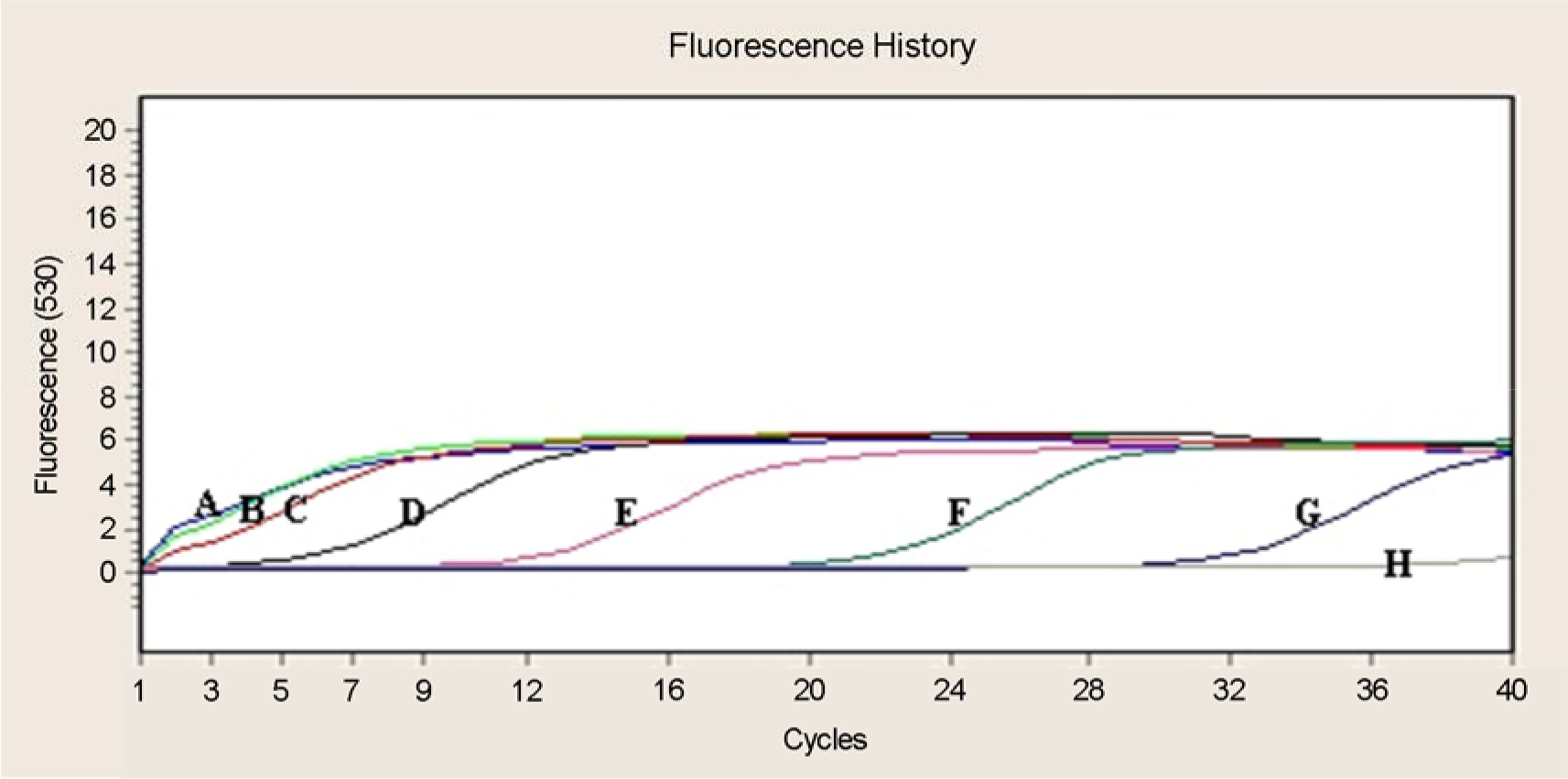
- In this study, new real-time PCR method based on the groEL gene was developed and investigated. Four spotted fever group (SFG) strains, four typhus group (TG) strains, and four scrub typhus group (STG) strains were easily differentiated as a distinct entity. This PCR assay was applied to detect Rickettsia DNA from 100 ticks. Twelve Haemaphysalis longicornis ticks were found positive and identified as spotted fever group Rickettsia. This real-time PCR method could simultaneously perform the rapid identification of rickettsiae and the differential diagnosis of SFG, TG, and STG in a single reaction.
Keyword
MeSH Terms
Figure
Reference
-
1). Chung HY. Rickettsial Infections. Infect. 17:89–92. 1985.2). Chung MH., Lee SH., Kim MJ., Lee JH., Kim ES., Kim MK., Park MY., Kang JS. Japanese spotted fever, South Korea. Emerg Infect Dis. 12:1122–1124. 2006.
Article3). Fournier PE., Roux V., Raoult D. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int J Syst Bacteriol. 48:839–849. 1998.
Article4). Kim KA., Lee SH., Jang WS., Oh MD., Kim I., Choe K. Two cases of tsutsugamushi disease in the spring. Korean J Infect Dis. 31:46–49. 1999.5). Lee JH., Park HS., Jang WJ., Koh SE., Kim JM., Shim SK., Park MY., Kim YW., Kim BJ., Kook YH., Park KH., Lee SH. Differentiation of rickettsiae by groEL gene analysis. J Clin Microbiol. 41:2952–2960. 2003.6). Lee JH., Park HS., Jung KD., Jang WJ., Koh SE., Kang SS., Lee IY., Lee WJ., Kim BJ., Kook YH., Park KH., Lee SH. Identification of the spotted fever group rickettsiae detected from Haemaphysalis longicornis in Korea. Microbiol Immunol. 47:301–304. 2003.7). Lee MK. Real-time polymerase chain reaction (PCR) in microbiology. Infect Chemother. 36:105–113. 2004.8). Marston EL., Sumner JW., Regnery RL. Evaluation of intraspecies genetic variation within the 60 kDa heat-shock protein gene (groEL) of Bartonella species. Int J Syst Bacteriol. 49:1015–1023. 1999.9). Raoult D., Dasch GA. Immunoblot cross-reactions among Rickettsia, Proteus spp. and Legionella spp. in patients with Mediterranean spotted fever. FEMS Immunol Med Microbiol. 11:13–18. 1995.10). Raoult D., Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev. 10:694–719. 1997.
Article11). Ririe KM., Rasmussen RP., Wittwer CT. Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal Biochem. 245:154–160. 1997.
Article12). Roux V., Fournier PE., Raoult D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J Clin Microbiol. 34:2058–2065. 1996.
Article13). Roux V., Raoult D. Phylogenetic analysis of the genus Rickettsia by 16S rDNA sequencing. Res Microbiol. 146:385–396. 1995.14). Roux V., Rydkina E., Eremeeva M., Raoult D. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int J Syst Bacteriol. 47:252–261. 1997.
Article15). Song HJ., Seong SY., Huh MS., Park SG., Jang WJ., Kee SH., Kim KH., Kim SC., Choi MS., Kim IS., Chang WH. Molecular and serologic survey of Orientia tsutsugamushi infection among field rodents in southern Cholla Province, Korea. Am J Trop Med Hyg. 58:513–518. 1998.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the Efficacies of Rapid Antigen Test, Multiplex PCR, and Real-time PCR for the Detection of a Novel Influenza A (H1N1) Virus
- Comparison of Rapid Antigen Test and Real-Time Reverse Transcription PCR for the Detection of Influenza B Virus
- Use of Immunochromatographic Assays for Identification of Mycobacterium tuberculosis Complex from Broth Cultures
- Multiplex Real-time Polymerase Chain Reaction Assays for Simultaneous Detection of
Vibrio cholerae ,Vibrio parahaemolyticus , andVibrio vulnificus - Understandings and Prospects of Laboratory Diagnosis of SARS-CoV-2