J Korean Orthop Assoc.
2008 Aug;43(4):420-427. 10.4055/jkoa.2008.43.4.420.
Motor Evoked Potential and Continuous Electromyography Monitoring during Spinal Surgery
- Affiliations
-
- 1Department of Orthopedic Surgery, Chungnam National University School of Medicine, Daejeon, Korea. jyyang@cnu.ac.kr
- KMID: 1480400
- DOI: http://doi.org/10.4055/jkoa.2008.43.4.420
Abstract
-
PURPOSE: A prospective study to determine the usefulness of the spinal cord monitoring (SCM) for predicting and preventing iatrogenic nerve injury during spinal surgery.
MATERIALS AND METHODS
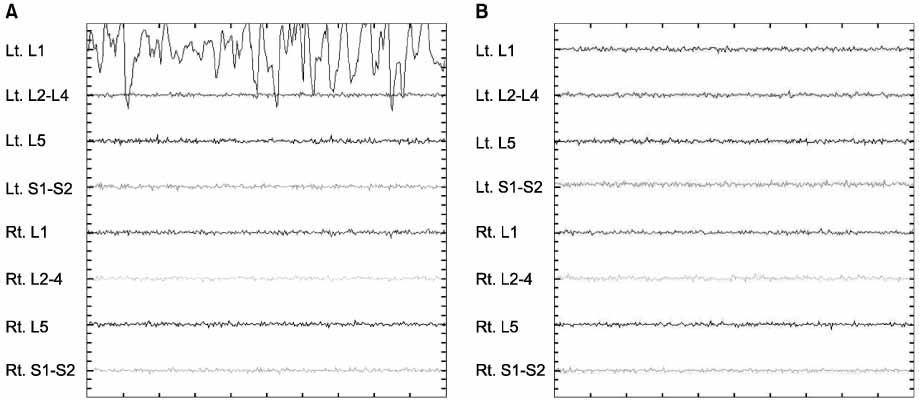
We studied 27 cases with adolescent idiopathic scoliosis (AIS, 7), degenerative spinal deformity (DSD, 13), and spinal stenosis (7) who received decompression and SCM including MEP and EMG. Intravenous anesthesia was performed using propofol. We initially could not measure SCM because of the presence of muscle relaxants. But later could check SCM in 24 cases. We evaluated the success rate of SCM, the degree of electrical stimulus, and abnormal signals.
RESULTS
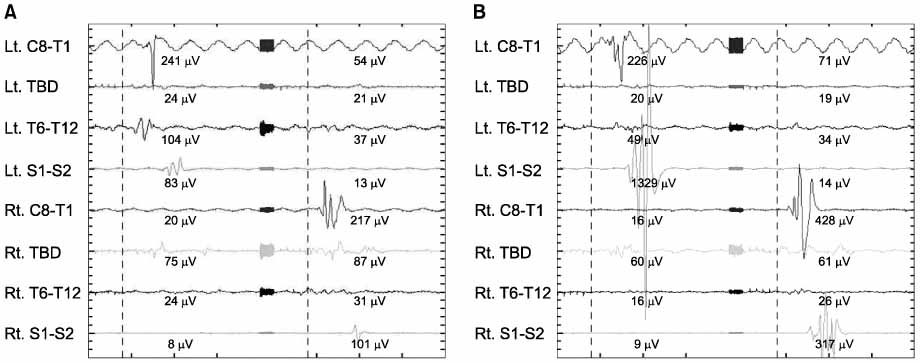
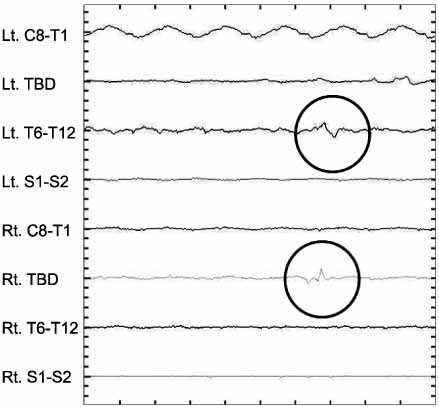
The success rate of SCM was 88.9%. MEP showed an increased stimulus indicating a normal neurologic response in: AIS (26.6%), DSD (24.3%), and spinal stenosis (15.7%). EMG showed abnormal signals in 4 cases. but 3 cases had no significant nerve damage. In one case, we reinserted the pedicle screw because we found nerve irritation by the probe. None of theses cases had neural deficits after the operation.
CONCLUSION
MEP and EMG allow for correction and decompression without spinal cord or nerve root injury with appropriate anesthesia technique and interpretation of abnormal signals required for SCM.
MeSH Terms
Figure
Reference
-
1. Bridwell KH, Lenke LG, Baldus C, Blanke K. Major intraoperative neurologic deficits in pediatric and adult spinal deformity patients. Incidence and etiology at one institution. Spine. 1998. 23:324–331.2. Calancie B, Harris W, Broton JG, Alexeeva N, Green BA. "Threshold-level" multipulse transcranial electrical stimulation of motor cortex for intraoperative monitoring of spinal motor tracts: description of method and comparison to somatosensory evoked potential monitoring. J Neurosurg. 1998. 88:457–470.
Article3. Cioni B, Meglio M, Rossi GF. Intraoperative motor evoked potentials monitoring in spinal neurosurgery. Arch Ital Biol. 1999. 137:115–126.4. Constantini S, Miller DC, Allen JC, Rorke LB, Freed D, Epstein FJ. Radical excision of intramedullary spinal cord tumors: surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg. 2000. 93:Suppl 2. S183–S193.
Article5. Cristante L, Herrmann HD. Surgical management of intramedullary spinal cord tumors: functional outcome and sources of morbidity. Neurosurgery. 1994. 35:69–76.6. Dawson GD. Cerebral responses to nerve stimulation in man. Br Med Bull. 1950. 6:326–329.
Article7. Jones SJ, Harrison R, Koh KF, Mendoza N, Crockard HA. Motor evoked potential monitoring during spinal surgery: responses of distal limb muscles to transcranial cortical stimulation with pulse trains. Electroencephalogr Clin Neurophysiol. 1996. 100:375–383.
Article8. Kalkman CJ, ten Brink SA, Been HD, Bovill JG. Variability of somatosensory cortical evoked potentials during spinal surgery. Effects of anesthetic technique and high-pass digital filtering. Spine. 1991. 16:924–929.
Article9. Langeloo DD, Lelivelt A, Louis Journée H, Slappendel R, de Kleuver M. Transcranial electrical motor-evoked potential monitoring during surgery for spinal deformity: a study of 145 patients. Spine. 2003. 28:1043–1050.10. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001. 83:1169–1181.11. Lesser RP, Raudzens P, Lüders H, et al. Postoperative neurological deficits may occur despite unchanged intraoperative somatosensory evoked potentials. Ann Neurol. 1986. 19:22–25.
Article12. Levy WJ, McCaffrey M, Hagichi S. Motor evoked potential as a predictor of recovery in chronic spinal cord injury. Neurosurgery. 1987. 20:138–142.
Article13. Lubicky JP, Spadaro JA, Yuan HA, Fredrickson BE, Henderson N. Variability of somatosensory cortical evoked potential monitoring during spinal surgery. Spine. 1989. 14:790–798.
Article14. Lyon R, Feiner J, Lieberman JA. Progressive suppression of motor evoked potentials during general anesthesia: the phenomenon of "anesthetic fade". J Neurosurg Anesthesiol. 2005. 17:13–19.15. Marsh B, White M, Morton N, Kenny GN. Pharmacokinetic model driven infusion of propofol in children. Br J Anaesth. 1991. 67:41–48.
Article16. Minto CF, Schnider TW, Egan TD, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology. 1997. 86:10–23.17. Nuwer MR. Spinal cord monitoring with somatosensory techniques. J Clin Neurophysiol. 1998. 15:183–193.
Article18. Nuwer MR, Dawson EG, Carlson LG, Kanim LE, Sherman JE. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995. 96:6–11.
Article19. Oro J, Haghighi SS. Effects of altering core body temperature on somatosensory and motor evoked potentials in rats. Spine. 1992. 17:498–503.
Article20. Pajewski TN, Arlet V, Phillips LH. Current approach on spinal cord monitoring: the point of view of the neurologist, the anesthesiologist and the spine surgeon. Eur Spine J. 2007. 16:Suppl 2. S115–S129.
Article21. Pelosi L, Lamb J, Grevitt M, Mehdian SM, Webb JK, Blumhardt LD. Combined monitoring of motor and somatosensory evoked potentials in orthopaedic spinal surgery. Clin Neurophysiol. 2002. 113:1082–1091.
Article22. Perlik SJ, VanEgeren R, Fisher MA. Somatosensory evoked potential surgical monitoring. Observations during combined isoflurane-nitrous oxide anesthesia. Spine. 1992. 17:273–276.
Article23. Quiñones-Hinojosa A, Lyon R, Zada G, et al. Changes in transcranial motor evoked potentials during intramedullary spinal cord tumor resection correlate with postoperative motor function. Neurosurgery. 2005. 56:982–993.24. Sakamoto T, Kawaguchi M, Kakimoto M, Inoue S, Takahashi M, Furuya H. The effect of hypothermia on myogenic motor-evoked potentials to electrical stimulation with a single pulse and a train of pulses under propofol/ketamine/fentanyl anesthesia in rabbits. Anesth Analg. 2003. 96:1692–1697.
Article25. York DH, Watts C, Raffensberger M, Spagnolia T, Joyce C. Utilization of somatosensory evoked cortical potentials in spinal cord injury. Prognostic limitations. Spine. 1983. 8:832–839.26. Yuan HA, Garfin SR, Dickman CA, Mardjetko SM. A historical cohort study of pedicle screw fixation in thoracic, lumbar, and sacral spinal fusions. Spine. 1994. 19:Suppl 20. S2279–S2296.27. Zornow MH, Grafe MR, Tybor C, Swenson MR. Preservation of evoked potentials in a case of anterior spinal artery syndrome. Electroencephalogr Clin Neurophysiol. 1990. 77:137–139.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intraoperative Neurophysiologic Monitoring: Basic Principles and Recent Update
- Intraoperative Monitoring of Hypoglossal Nerve Using Hypoglossal Motor Evoked Potential in Infratentorial Tumor Surgery: A Report of Two Cases
- Intraoperative Monitoring for Tethered Cord Syndrome Using Somatosensory Evoked Potential and Motor Evoked Potential: Report of three cases
- Intraoperative Neurophysiology Monitoring for Spinal Dysraphism
- Anesthetic experience performing intraoperative monitoring of motor evoked potentials during scoliosis surgery in adolescent patients: report on 7 cases: Seven cases report