J Korean Soc Transplant.
2010 Sep;24(3):187-195. 10.4285/jkstn.2010.24.3.187.
Infectious Complications in Renal Transplant Recipients: Changing Epidemiology under Modern Immunosuppression
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea. jwhamd@snu.ac.kr
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 3Transplantation Research Institute, Seoul National University Medical Research Center, Seoul, Korea.
- 4Transplantation Research Institute, Seoul National University Hospital, Seoul, Korea.
- KMID: 1464312
- DOI: http://doi.org/10.4285/jkstn.2010.24.3.187
Abstract
- BACKGROUND
Immunosuppressive agents with higher potencies, such as tacrolimus and mycophenolate mofetil (MMF), have been introduced and widely accepted in clinical practice. This study evaluated the impact of these newer immunosuppressive drugs on the pattern and timing of post-kidney transplantation infections.
METHODS
Data of kidney transplant recipients at the Seoul National University Hospital between January 1990 and November 2005 were analyzed. Recipients were divided into double immunosuppression (double group, n=198), triple immunosuppression including MMF (MMF group, n=253), and azathioprine (AZA, n=184) groups.
RESULTS
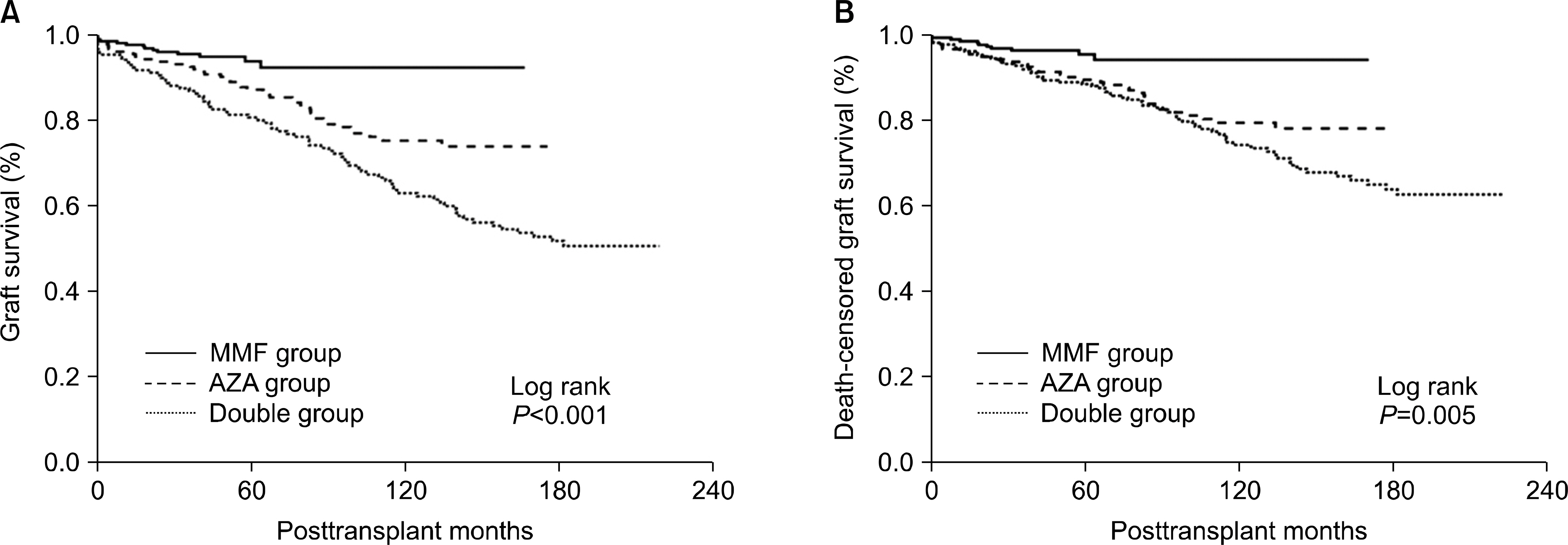
The MMF group demonstrated higher graft survival and reduced rates of acute rejection within the fifth post-transplant year than both the AZA (P<0.001) and the double (P<0.001) groups. The overall incidence of infection in the first month was significantly higher in the MMF group (2.17/1,000 transplant-days) than in the AZA (0.73/1,000 transplant-days) and double (0.84/1,000 transplant-days) groups (P=0.01, ANOVA), and this was caused by viral infections that were significantly higher in the MMF (1.57/1,000 transplant-days) group than in the AZA (0.54/1,000 transplant-days) and double (0.67/1,000 transplant-days) groups. MMF was identified as a significant risk factor for viral infection (P=0.013; OR, 2.04; 95% CI, 1.16-3.60) in a multivariate logistic regression analysis.
CONCLUSIONS
The results suggest that viral infection rates were higher in the MMF group and should be considered the primary source of perioperative infectious complications in MMF-receiving recipients.
Keyword
MeSH Terms
Figure
Reference
-
References
1). Meier-Kriesche HU, Li ddS, Gruessner RW, Fung JJ, Bustami RT, Barr ML, et al. Immunosuppression: evolution in practice and trends, 1994–2004. Am J Transplant. 2006; 6:1111–31.
Article2). Kainz A, Heinze G, Korbély R, Schwarz C, Oberbauer R. Mycophenolate mofetil use is associated with prolonged graft survival after kidney transplantation. Transplantation. 2009; 88:1095–100.
Article3). Chang SH, Russ GR, Chadban SJ, Campbell SB, McDonald SP. Trends in kidney transplantation in Australia and New Zealand, 1993–2004. Transplantation. 2007; 84:611–8.
Article4). Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: metaanalysis and meta-regression of randomised trial data. BMJ. 2005; 331:810.
Article5). Opelz G, Döhler B. Collaborative Transplant Study. Influence of immunosuppressive regimens on graft survival and secondary outcomes after kidney transplantation. Transplantation. 2009; 87:795–802.
Article6). Halloran P, Mathew T, Tomlanovich S, Groth C, Hooftman L, Barker C. Mycophenolate mofetil in renal allograft recipients: a pooled efficacy analysis of three randomized, double-blind, clinical studies in prevention of rejection. The International Mycophenolate Mofetil Renal Transplant Study Groups. Transplantation. 1997; 63:39–47. Erratum in: Transplantation 1997;63: 618.7). Srinivas TR, Kaplan B, Schold JD, Meier-Kriesche HU. The impact of mycophenolate mofetil on longterm outcomes in kidney transplantation. Transplantation. 2005; 80(2 Suppl):S211–20.
Article8). Simon DM, Levin S. Infectious complications of solid organ transplantations. Infect Dis Clin North Am. 2001; 15:521–49.
Article9). Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007; 357:2601–14.
Article10). Husain S, Singh N. The impact of novel immunosuppressive agents on infections in organ transplant recipients and the interactions of these agents with antimicrobials. Clin Infect Dis. 2002; 35:53–61.
Article11). Humar A, Michaels M. AST ID Working Group on Infectious Disease Monitoring. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant. 2006; 6:262–74.
Article12). Patel R, Paya CV. Infections in solid-organ transplant recipients. Clin Microbiol Rev. 1997; 10:86–124.
Article13). San Juan R, Aguado JM, Lumbreras C, Diaz-Pedroche C, Lopez-Medrano F, Lizasoain M, et al. Incidence, clinical characteristics and risk factors of late infection in solid organ transplant recipients: data from the RESITRA study group. Am J Transplant. 2007; 7:964–71.
Article14). Parasuraman R, Yee J, Karthikeyan V, del Busto R. Infectious complications in renal transplant recipients. Adv Chronic Kidney Dis. 2006; 13:280–94.
Article15). Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998; 338:1741–51.
Article16). Pourfarziani V, Panahi Y, Assari S, Moghani-Lankarani M, Saadat SH. Changing treatment protocol from azathioprine to mycophenolate mofetil: decrease in renal dysfunction, increase in infections. Transplant Proc. 2007; 39:1237–40.
Article17). Browne BJ. The tricontinental mycophenolate mofetil trial. Transplantation. 1996; 62:1697.
Article18). Knight SR, Russell NK, Barcena L, Morris PJ. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review. Transplantation. 2009; 87:785–94.
Article19). Hanvesakul R, Kubal C, Jham S, Sarkar E, Eardley K, Adu D, et al. Increased incidence of infections following the late introduction of mycophenolate mofetil in renal transplant recipients. Nephrol Dial Transplant. 2008; 23:4049–53.
Article20). Zafrani L, Truffaut L, Kreis H, Etienne D, Rafat C, Lechaton S, et al. Incidence, risk factors and clinical consequences of neutropenia following kidney transplantation: a retrospective study. Am J Transplant. 2009; 9:1816–25.
Article21). Rubin RH, Wolfson JS, Cosimi AB, Tolkoff-Rubin NE. Infection in the renal transplant recipient. Am J Med. 1981; 70:405–11.
Article22). Kim YK, Kim DW. Seroepidemiology of human cytomegalovirus in healthy adults measured by means of the anti-complement immunofluorescence technique. Korean J Infect Dis. 1992; 24:87–92. (김유겸, 유대원. Anticomplement Immunofluorescence 검사법을 이용한 건강한 성인의 Human Cytomegalovirus에 대한 항체가의 조사 연구. 감염 1992;24: 87–92.).23). Kidney Disease. Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009; 9(Suppl 3):S1–155.24). Alangaden GJ, Thyagarajan R, Gruber SA, Morawski K, Garnick J, El-Amm JM, et al. Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clin Transplant. 2006; 20:401–9.
Article25). Jorge S, Guerra J, Santana A, Mil-Homens C, Prata MM. Mycophenolate mofetil: ten years' experience of a renal transplant unit. Transplant Proc. 2008; 40:700–4.
Article26). Moreso F, Seron D, Morales JM, Cruzado JM, Gil-Vernet S, Perez JL, et al. Incidence of leukopenia and cytomegalovirus disease in kidney transplants treated with mycophenolate mofetil combined with low cyclosporine and steroid doses. Clin Transplant. 1998; 12:198–205.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Squamous Cell Carcinomain Renal Transplant Recipient
- Cutaneous Manifestations of Kidney Transplant Recipients
- Malignant tumors in renal transplant recipients receiving longterm immunosuppression: Their treatment and prognosis
- Kidney Retransplantation
- Management of Opportunistic Infections after Organ Transplantation