Korean J Bone Metab.
2012 May;19(1):11-20. 10.11005/kjbm.2012.19.1.11.
Identification of Alternatively Spliced Forms of human OSCAR in Osteoclasts
- Affiliations
-
- 1Department of Pharmacology, Chonnam National University Medical School, Gwangju, Korea. nacksung@chonnam.ac.kr
- KMID: 1464231
- DOI: http://doi.org/10.11005/kjbm.2012.19.1.11
Abstract
OBJECTIVES
Osteoclasts are multinucleated giant cells which can resorb bone and differentiated from hematopoietic cells. We have previously reported murine osteoclast-associated receptor (OSCAR) may be an important bone-specific regulator of osteoclast differentiation. We have cloned soluble form of human OSCAR (hOSCAR) and examined the role of hOSCAR on osteoclast differentiation.
METHODS
Osteoclast differentiation was induced by treatment with macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa B ligand (RANKL) and tartrate-resistant acid phosphatase (TRAP) staining and pit formation were performed. Expression was measured by flow cytometry analysis, Northern and Western blot analysis.
RESULTS
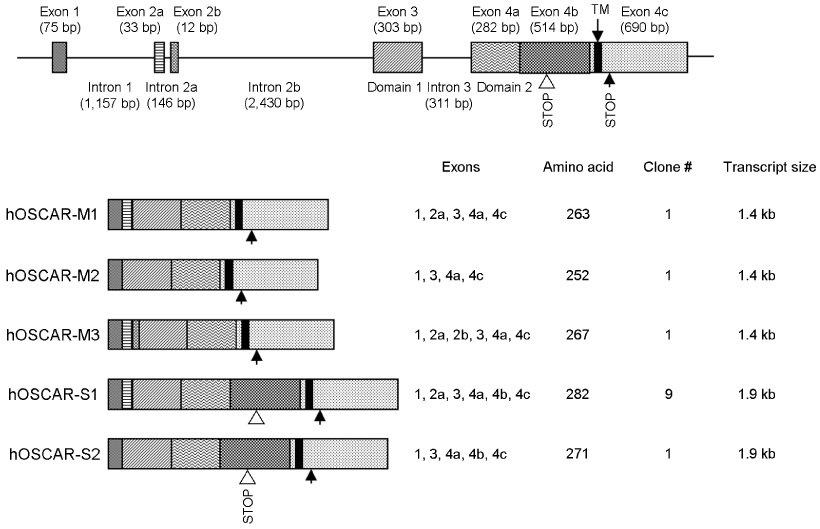
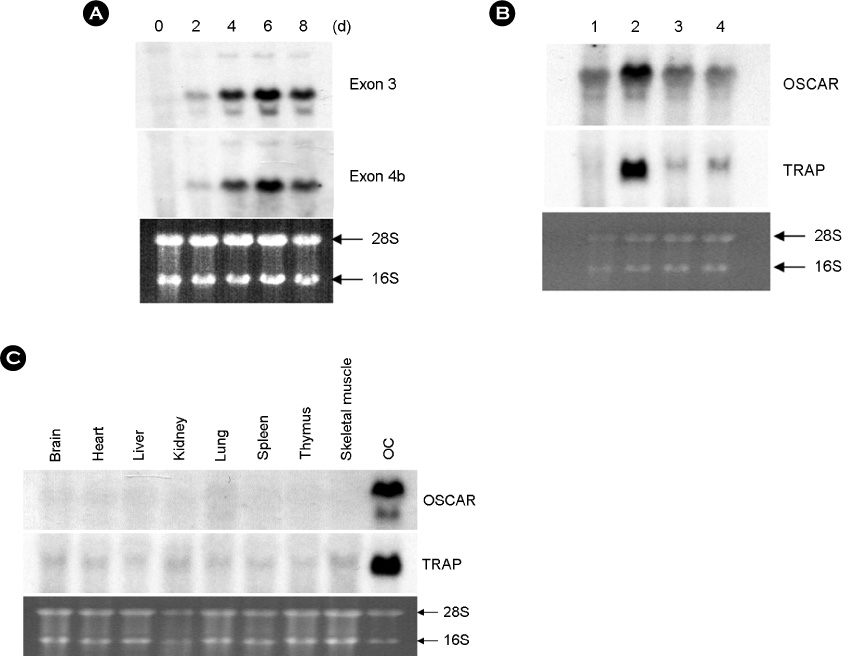
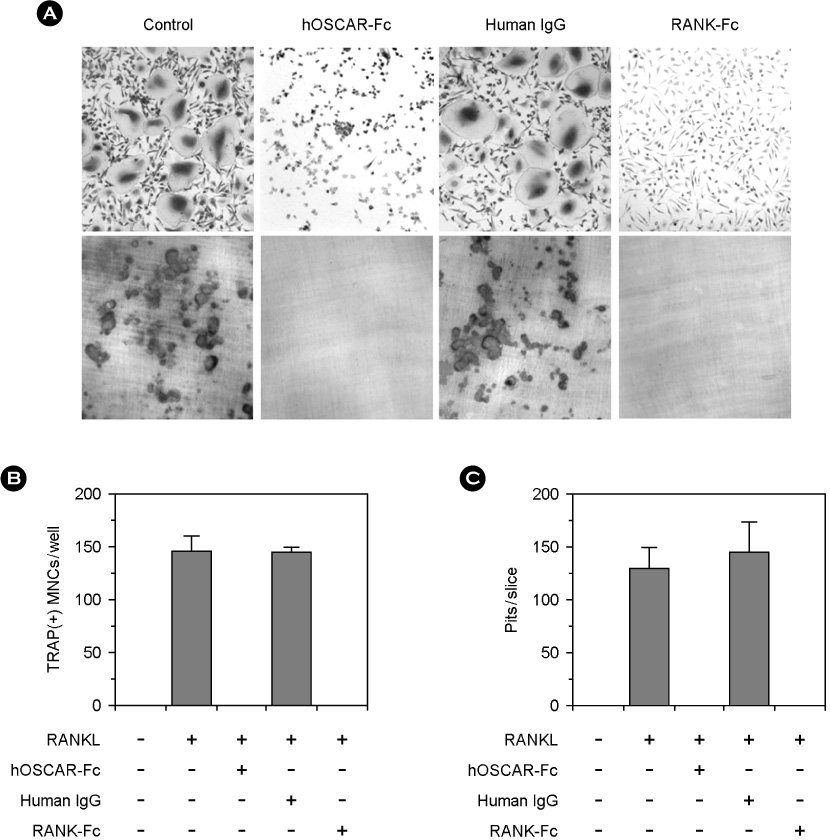
hOSCAR is expressed in osteoclast cells and involved in the differentiation of osteoclasts from peripheral blood mononuclear cells (PBMC). Two alternatively spliced forms (soluble hOSCAR [hOSCAR-S]) of hOSCAR were identified from osteoclasts complementary deoxyribonucleic acid (cDNA) library derived from PBMC. Putative transmembrane domain was not found in hOSCAR-S forms and it suggested that these forms might be secreted from osteoclast cells. These secreted forms of hOSCAR attenuated RANKL-induced osteoclast formation and bone resorption.
CONCLUSIONS
Human osteoclasts express at least five different OSCAR messenger ribonucleic acid (mRNA) isoforms which could play different regulatory roles for differentiation. The secreted forms of hOSCAR might be a negative regulator of membrane-bounded forms of OSCAR.
Keyword
MeSH Terms
Figure
Reference
-
1. Walsh MC, Kim N, Kadono Y, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006. 24:33–63.
Article2. Marks SC Jr, Lane PW. Osteopetrosis, a new recessive skeletal mutation on chromosome 12 of the mouse. J Hered. 1976. 67:11–18.
Article3. Wong BR, Josien R, Lee SY, et al. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997. 186:2075–2080.
Article4. Anderson DM, Maraskovsky E, Billingsley WL, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997. 390:175–179.
Article5. Yasuda H, Shima N, Nakagawa N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998. 95:3597–3602.
Article6. Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998. 93:165–176.
Article7. Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999. 20:345–357.
Article8. Koga T, Inui M, Inoue K, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004. 428:758–763.
Article9. Ishida N, Hayashi K, Hoshijima M, et al. Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J Biol Chem. 2002. 277:41147–41156.
Article10. Ishikawa S, Arase N, Suenaga T, et al. Involvement of FcRgamma in signal transduction of osteoclast-associated receptor (OSCAR). Int Immunol. 2004. 16:1019–1025.
Article11. Herman S, Müller RB, Krönke G, et al. Induction of osteoclast-associated receptor, a key osteoclast costimulation molecule, in rheumatoid arthritis. Arthritis Rheum. 2008. 58:3041–3050.
Article12. Merck E, de Saint-Vis B, Scuiller M, et al. Fc receptor gamma-chain activation via hOSCAR induces survival and maturation of dendritic cells and modulates Toll-like receptor responses. Blood. 2005. 105:3623–3632.
Article13. Merck E, Gaillard C, Gorman DM, et al. OSCAR is an FcRgamma-associated receptor that is expressed by myeloid cells and is involved in antigen presentation and activation of human dendritic cells. Blood. 2004. 104:1386–1395.
Article14. Merck E, Gaillard C, Scuiller M, et al. Ligation of the FcR gamma chain-associated human osteoclast-associated receptor enhances the proinflammatory responses of human monocytes and neutrophils. J Immunol. 2006. 176:3149–3156.
Article15. Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994. 179:1109–1118.
Article16. Kim N, Takami M, Rho J, Josien R, Choi Y. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J Exp Med. 2002. 195:201–209.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of HuD (a paraneoplastic encephalomyelitis antigen) mRNA in lung cancer
- Temporal Patterns of Expression of Type II Procollagen Splice Forms During the Chondrogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells
- Identification and functional characterization of an alternative splice variant within the fourth exon of human nanog
- An alternatively spliced form of Met receptor is tumorigenic
- YBX1 Promotes the Inclusion of RUNX2 Alternative Exon 5 in Dental Pulp Stem Cells