Korean J Physiol Pharmacol.
2011 Aug;15(4):189-194. 10.4196/kjpp.2011.15.4.189.
Increased Expression of ATP-sensitive K+ Channels Improves the Right Ventricular Tolerance to Hypoxia in Rabbit Hearts
- Affiliations
-
- 1National Research Laboratory for Mitochondrial Signaling, Department of Physiology, College of Medicine, Cardiovascular and Metabolic Disease Center, Inje University, Busan 614-735, Korea. phyhanj@inje.ac.kr
- 2Department of Sport and Leisure Studies, Andong Science College, Andong 760-709, Korea.
- 3Department of Neuropsychiatry, College of Medicine, Paik Inje Memorial Clinical Research Institute, Inje University, Busan 614-735, Korea.
- 4Department of Physiology, School of Medicine, Kangwon National University, Chuncheon 200-701, Korea.
- 5Department of Physiology & Chronic Disease Research Center, Keimyung University School of Medicine, Daegu 700-712, Korea.
- KMID: 1448923
- DOI: http://doi.org/10.4196/kjpp.2011.15.4.189
Abstract
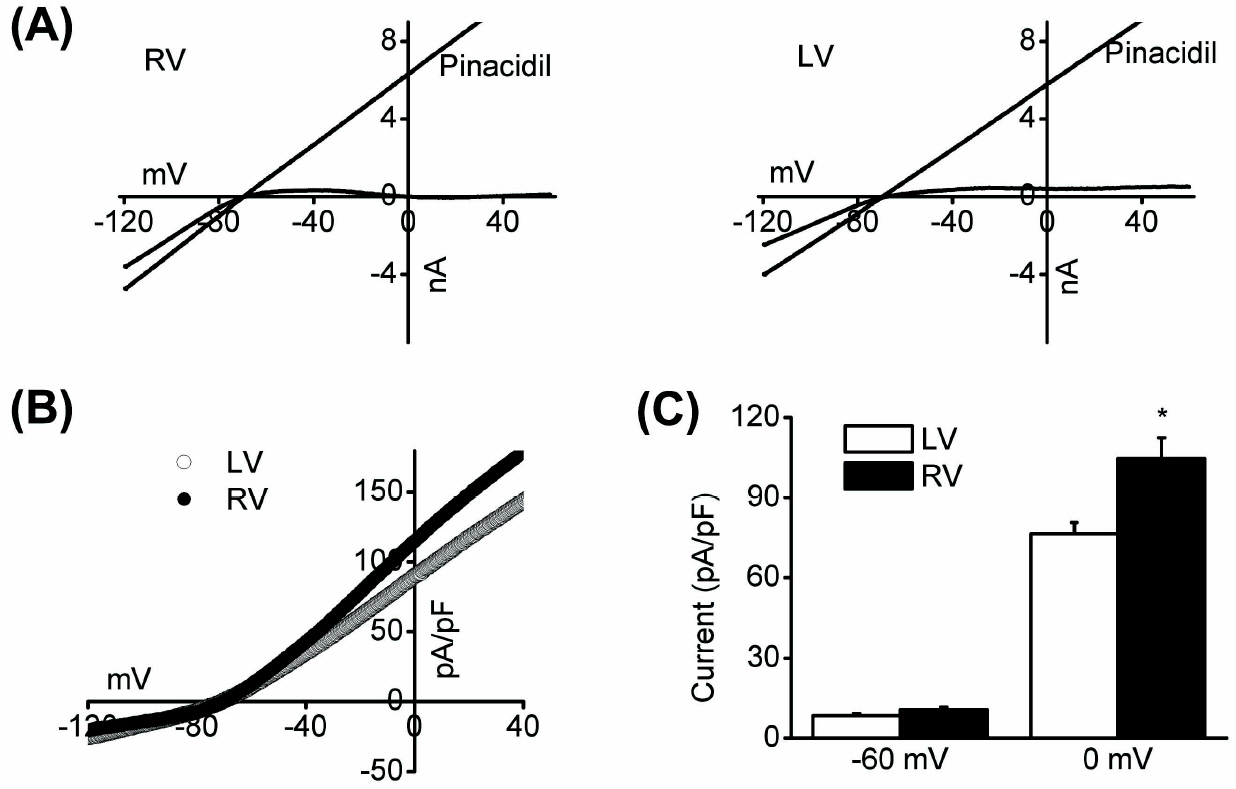
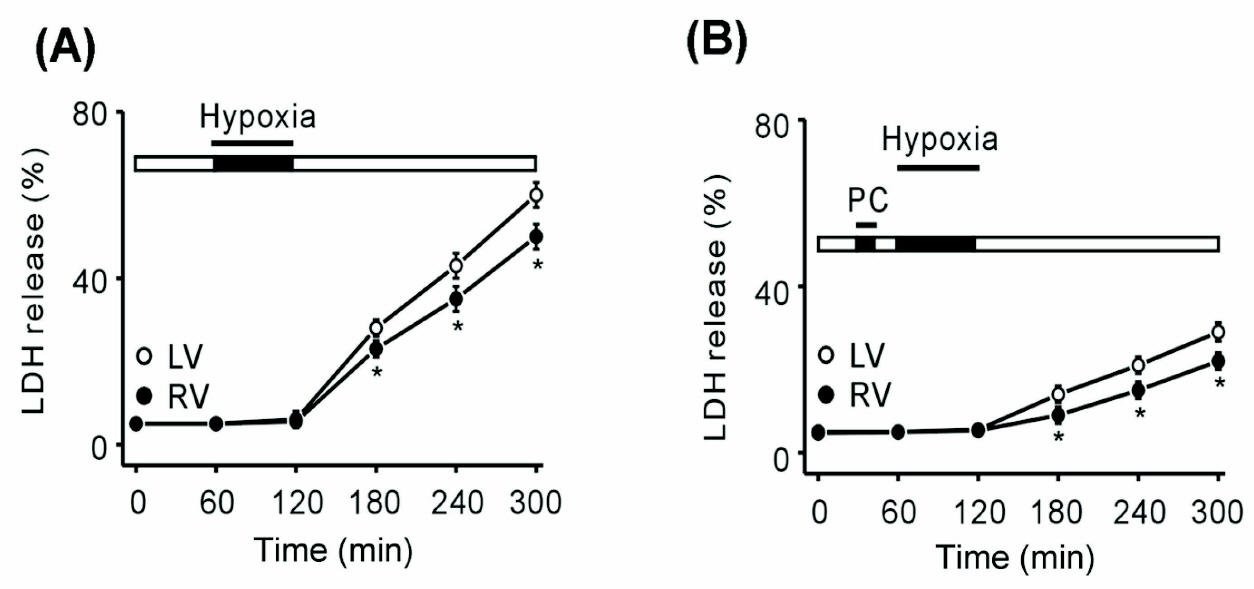
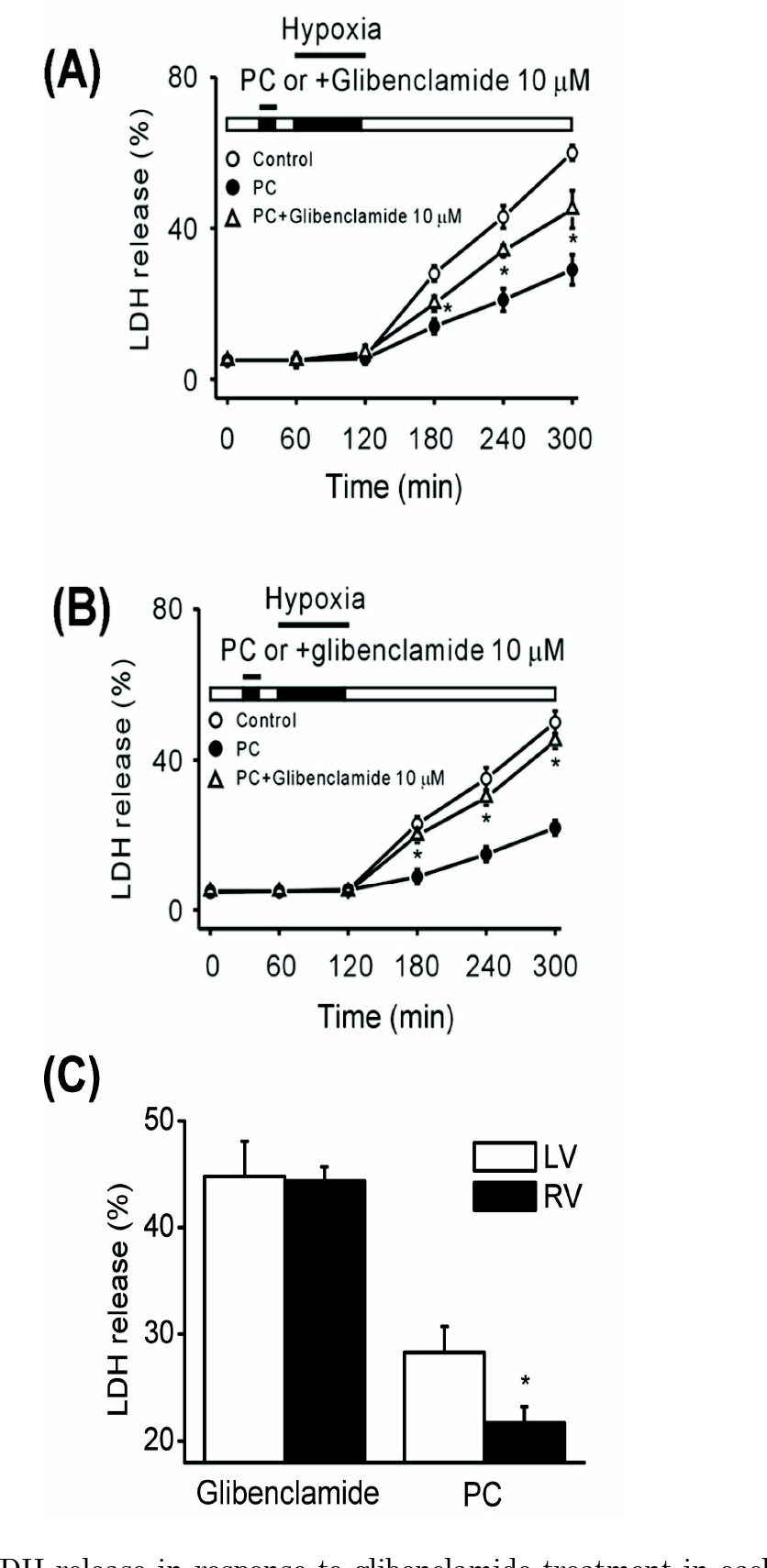
- ATP-sensitive K+ channels (KATP) are major component of preventing ischemia-reperfusion injury. However, there is little information regarding to the expressional difference of K(ATP) and its function between left and right ventricles. In this study, we measured the lactate dehydrogenase release of rabbit heart slices in vitro and determined the difference of the K(ATP) expression at the both ventricles by measuring the level of K(ATP)-forming Kir6.2 (OcKir6.2) mRNA using in situ hybridization. The hearts were preconditioned with 15 min hypoxia and reoxygenated for 15 min before a hypoxic period of 60 min, followed by reoxygenation for 180 min. With hypoxic preconditioning (100% N2) with 15 min, left ventricles (LV) showed higher release of LDH comparing with right ventricles (RV). Adding KATP blocker glibenclamide (10 microM) prior to a hypoxic period of 60 min, hypoxic preconditioning effect of RV was more abolished than LV. With in situ hybridization, the optical density of OcKir6.2 was higher in RV. Therefore, we suggest that different K(ATP) expression between LV and RV is responsible for the different response to hypoxia and hypoxic preconditioning of rabbit hearts.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Acute Hypoxia Activates an ENaC-like Channel in Rat Pheochromocytoma (PC12) Cells
Yeon Ju Bae, Jae-Cheal Yoo, Nammi Park, Dawon Kang, Jaehee Han, Eunmi Hwang, Jae-Yong Park, Seong-Geun Hong
Korean J Physiol Pharmacol. 2013;17(1):57-64. doi: 10.4196/kjpp.2013.17.1.57.
Reference
-
References
1. Podesser B, Wollenek G, Seitelberger R, Siegel H, Wolner E, Firbas W, Tschabitscher M. Epicardial branches of the coronary arteries and their distribution in the rabbit heart: the rabbit heart as a model of regional ischemia. Anat Rec. 1997; 247:521–527.
Article2. Baker JE, Holman P, Gross GJ. Preconditioning in immature rabbit hearts: role of KATP channels. Circulation. 1999; 99:1249–1254.3. Kloner RA, Bolli R, Marban E, Reinlib L, Braunwald E. Medical and cellular implications of stunning, hibernation, and preconditioning: an NHLBI workshop. Circulation. 1998; 97:1848–1867.4. Auchampach JA, Grover GJ, Gross GJ. Blockade of ischaemic preconditioning in dogs by the novel ATP dependent potassium channel antagonist sodium 5-hydroxydecanoate. Cardiovasc Res. 1992; 26:1054–1062.
Article5. Gross GJ, Peart JN. KATP channels and myocardial preconditioning: an update. Am J Physiol Heart Circ Physiol. 2003; 285:H921–930.
Article6. Gross GJ. ATP-sensitive potassium channels and myocardial preconditioning. Basic Res Cardiol. 1995; 90:85–88.
Article7. Seharaseyon J, Sasaki N, Ohler A, Sato T, Fraser H, Johns DC, O'Rourke B, Marbán E. Evidence against functional heteromultimerization of the KATP channel subunits Kir6.1 and Kir6.2. J Biol Chem. 2000; 275:17561–17565.8. Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci U S A. 2002; 99:13278–13283.
Article9. Park SW, Lee SK, Kim JM, Yoon JS, Kim YH. Effects of quetiapine on the brain-derived neurotrophic factor expression in the hippocampus and neocortex of rats. Neurosci Lett. 2006; 402:25–29.
Article10. Han J, Kim N, Joo H, Kim E, Earm YE. ATP-sensitive K(+) channel activation by nitric oxide and protein kinase G in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol. 2002; 283:H1545–1554.11. Lee KW, Norell MS. Cardiogenic shock complicating myocardial infarction and outcome following percutaneous coronary intervention. Acute Card Care. 2008; 10:131–143.
Article12. Hochman JS, Buller CE, Sleeper LA, Boland J, Dzavik V, Sanborn TA, Godfrey E, White HD, Lim J, LeJemtel T. Cardiogenic shock complicating acute myocardial infarction-etiologies, management and outcome: a report from the shock trial registry. should we emergently revascularize occluded Coronaries for cardiogenic shock? J Am Coll Cardiol. 2000; 36(3 Suppl A):1063–1070.13. Brodie BR, Stuckey TD, Hansen C, Bradshaw BH, Downey WE, Pulsipher MW. Comparison of late survival in patients with cardiogenic shock due to right ventricular infarction versus left ventricular pump failure following primary percutaneous coronary intervention for ST-elevation acute myocardial infarction. Am J Cardiol. 2007; 99:431–435.
Article14. Shuhaiber HJ, Juggi JS, John V, Yousof AM, Braveny P. Differences in the recovery of right and left ventricular function after ischaemic arrest and cardioplegia. Eur J Cardiothorac Surg. 1990; 4:435–440.
Article15. Mehta SR, Eikelboom JW, Natarajan MK, Diaz R, Yi C, Gibbons RJ, Yusuf S. Impact of right ventricular involvement on mortality and morbidity in patients with inferior myocardial infarction. J Am Coll Cardiol. 2001; 37:37–43.16. Goldstein JA. Right versus left ventricular shock: a tale of two ventricles. J Am Coll Cardiol. 2003; 41:1280–1282.17. Kinch JW, Ryan TJ. Right ventricular infarction. N Engl J Med. 1994; 330:1211–1217.
Article18. Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983; 305:147–148.19. Grover GJ, Sleph PG, Dzwonczyk S. Role of myocardial ATP-sensitive potassium channels in mediating preconditioning in the dog heart and their possible interaction with adenosine A1-receptors. Circulation. 1992; 86:1310–1316.
Article20. Gross GJ, Auchampach JA. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res. 1992; 70:223–233.
Article21. Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marbán E, Nakaya H. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002; 109:509–516.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effects of intracellular monocarboxylates on the ATP-sensitive potassium channels in rabbit ventricular myocytes
- The Effect of Trifluoroacetic Acid, a Metabolite of Isoflurane on the ATP-sensitive Potassium Channel in Rabbit Ventricular Myocytes
- Protein Kinase C Activates ATP-sensitive Potassium Channels in Rabbit Ventricular Myocytes
- Activation of ATP-sensitive potassium channels by the predominant metabolite of isoflurane in rabbit ventricular myocytes
- Effects of micron-Opioid Agonist on ATP-sensitive Potassium Channel Activity in Isolated Ventricular Cardiomyocytes