Anat Cell Biol.
2011 Dec;44(4):265-273. 10.5115/acb.2011.44.4.265.
Laminar flow activation of ERK5 leads to cytoprotective effect via CHIP-mediated p53 ubiquitination in endothelial cells
- Affiliations
-
- 1Department of Biology and Center for Inflammation, Immunity and Infection, Georgia State University, Atlanta, GA, USA.
- 2Department of Pharmacology and Aging-associated Vascular Disease Research Center, Yeungnam University College of Medicine, Daegu, Korea. Changhoon_Woo@yu.ac.kr
- KMID: 1447440
- DOI: http://doi.org/10.5115/acb.2011.44.4.265
Abstract
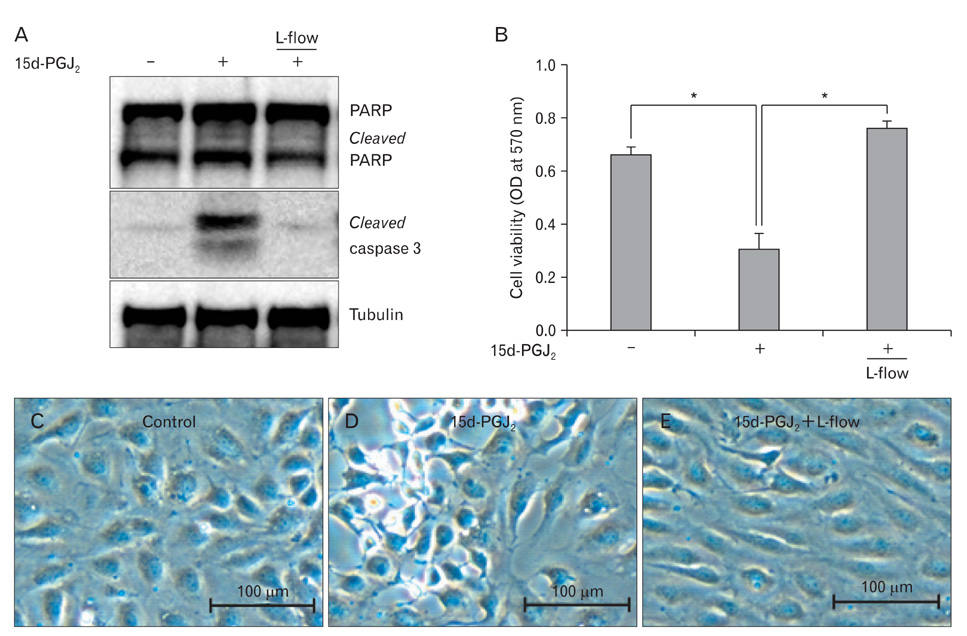
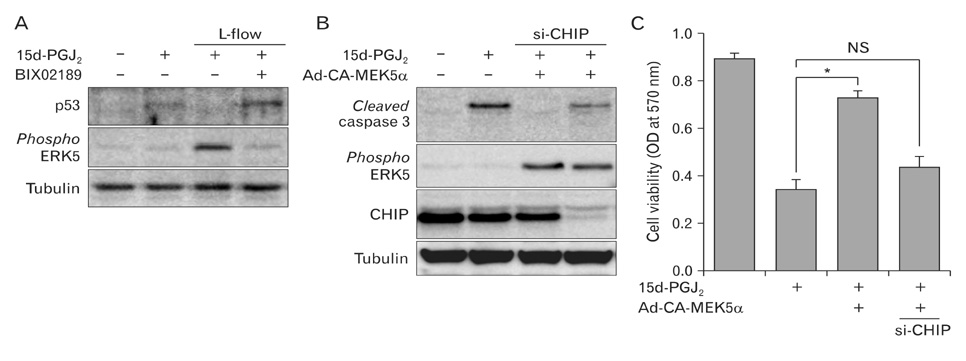
- Atherosclerosis is readily observed in areas where disturbed flow is formed, while the atheroprotective region is found in areas with steady laminar flow (L-flow). It has been established that L-flow protects endothelial cells against endothelial dysfunction, including apoptosis and inflammation. It has also been reported that extracellular signal-regulated kinase 5 (ERK5) regulated endothelial integrity and protected endothelial cells from vascular dysfunction and disease under L-flow. However, the molecular mechanism by which L-flow-induced ERK5 activation inhibits endothelial apoptosis has not yet been determined. Transcription factor p53 is a major pro-apoptotic factor which contributes to apoptosis in various cell types. In this study, we found that 15-deoxy-Delta(12,14)-prostaglandin J2 induced p53 expression and that endothelial apoptosis was reduced under the L-flow condition. This anti-apoptotic response was reversed by the biochemical inhibition of ERK5 activation. It was also found that activation of ERK5 protected endothelial apoptosis in a C terminus of Hsc70-interacting protein (CHIP) ubiquitin ligase-dependent manner. Moreover, molecular interaction between ERK5-CHIP and p53 ubiquitination were addressed with a CHIP ubiquitin ligase activity assay. Taken together, our data suggest that the ERK5-CHIP signal module elicited by L-flow plays an important role in the anti-apoptotic mechanism in endothelial cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Deguchi JO, Aikawa M, Tung CH, Aikawa E, Kim DE, Ntziachristos V, Weissleder R, Libby P. Inflammation in atherosclerosis: visualizing matrix metalloproteinase action in macrophages in vivo. Circulation. 2006. 114:55–62.2. Libby P. Inflammation in atherosclerosis. Nature. 2002. 420:868–874.3. Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999. 282:2035–2042.4. Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, García-Cardeña G, Gimbrone MA Jr. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci USA. 2004. 101:14871–14876.5. Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA Jr, García-Cardeña G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006. 116:49–58.6. Heo KS, Lee H, Nigro P, Thomas T, Le NT, Chang E, McClain C, Reinhart-King CA, King MR, Berk BC, Fujiwara K, Woo CH, Abe J. PKCζ mediates disturbed flow-induced endothelial apoptosis via p53 SUMOylation. J Cell Biol. 2011. 193:867–884.7. Yoshizumi M, Abe J, Tsuchiya K, Berk BC, Tamaki T. Stress and vascular responses: atheroprotective effect of laminar fluid shear stress in endothelial cells: possible role of mitogen-activated protein kinases. J Pharmacol Sci. 2003. 91:172–176.8. Akaike M, Che W, Marmarosh NL, Ohta S, Osawa M, Ding B, Berk BC, Yan C, Abe J. The hinge-helix 1 region of peroxisome proliferator-activated receptor gamma1 (PPARgamma1) mediates interaction with extracellular signal-regulated kinase 5 and PPARgamma1 transcriptional activation: involvement in flow-induced PPARgamma activation in endothelial cells. Mol Cell Biol. 2004. 24:8691–8704.9. Buschbeck M, Ullrich A. The unique C-terminal tail of the mitogen-activated protein kinase ERK5 regulates its activation and nuclear shuttling. J Biol Chem. 2005. 280:2659–2667.10. Suzaki Y, Yoshizumi M, Kagami S, Koyama AH, Taketani Y, Houchi H, Tsuchiya K, Takeda E, Tamaki T. Hydrogen peroxide stimulates c-Src-mediated big mitogen-activated protein kinase 1 (BMK1) and the MEF2C signaling pathway in PC12 cells: potential role in cell survival following oxidative insults. J Biol Chem. 2002. 277:9614–9621.11. Regan CP, Li W, Boucher DM, Spatz S, Su MS, Kuida K. Erk5 null mice display multiple extraembryonic vascular and embryonic cardiovascular defects. Proc Natl Acad Sci USA. 2002. 99:9248–9253.12. Pi X, Yan C, Berk BC. Big mitogen-activated protein kinase (BMK1)/ERK5 protects endothelial cells from apoptosis. Circ Res. 2004. 94:362–369.13. Garin G, Abe J, Mohan A, Lu W, Yan C, Newby AC, Rhaman A, Berk BC. Flow antagonizes TNF-alpha signaling in endothelial cells by inhibiting caspase-dependent PKC zeta processing. Circ Res. 2007. 101:97–105.14. Taketa K, Matsumura T, Yano M, Ishii N, Senokuchi T, Motoshima H, Murata Y, Kim-Mitsuyama S, Kawada T, Itabe H, Takeya M, Nishikawa T, Tsuruzoe K, Araki E. Oxidized low density lipoprotein activates peroxisome proliferator-activated receptor-alpha (PPARalpha) and PPARgamma through MAPK-dependent COX-2 expression in macrophages. J Biol Chem. 2008. 283:9852–9862.15. Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995. 83:803–812.16. Uchida K, Shibata T. 15-Deoxy-Delta(12,14)-prostaglandin J2: an electrophilic trigger of cellular responses. Chem Res Toxicol. 2008. 21:138–144.17. Bishop-Bailey D, Hla T. Endothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxy-Delta12, 14-prostaglandin J2. J Biol Chem. 1999. 274:17042–17048.18. Dong YG, Chen DD, He JG, Guan YY. Effects of 15-deoxy-delta12,14-prostaglandin J2 on cell proliferation and apoptosis in ECV304 endothelial cells. Acta Pharmacol Sin. 2004. 25:47–53.19. Erl W, Weber C, Zernecke A, Neuzil J, Vosseler CA, Kim HJ, Weber PC. Cyclopentenone prostaglandins induce endothelial cell apoptosis independent of the peroxisome proliferatoractivated receptor-gamma. Eur J Immunol. 2004. 34:241–250.20. Vosseler CA, Erl W, Weber PC. Structural requirements of cyclopentenone prostaglandins to induce endothelial cell apoptosis. Biochem Biophys Res Commun. 2003. 307:322–326.21. Ikai K, Kudo H, Toda K, Fukushima M. Induction of apoptosis, p53 and heme oxygenase-1 by cytotoxic prostaglandin delta12-PGJ2 in transformed endothelial cells. Prostaglandins Leukot Essent Fatty Acids. 1998. 58:295–300.22. Ho TC, Chen SL, Yang YC, Chen CY, Feng FP, Hsieh JW, Cheng HC, Tsao YP. 15-deoxy-Delta(12,14)-prostaglandin J2 induces vascular endothelial cell apoptosis through the sequential activation of MAPKS and p53. J Biol Chem. 2008. 283:30273–30288.23. Esser C, Scheffner M, Höhfeld J. The chaperone-associated ubiquitin ligase CHIP is able to target p53 for proteasomal degra dation. J Biol Chem. 2005. 280:27443–27448.24. Fuchs SY, Adler V, Buschmann T, Wu X, Ronai Z. Mdm2 association with p53 targets its ubiquitination. Oncogene. 1998. 17:2543–2547.25. Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997. 420:25–27.26. Woo CH, Le NT, Shishido T, Chang E, Lee H, Heo KS, Mickelsen DM, Lu Y, McClain C, Spangenberg T, Yan C, Molina CA, Yang J, Patterson C, Abe J. Novel role of C terminus of Hsc70-interacting protein (CHIP) ubiquitin ligase on inhibiting cardiac apoptosis and dysfunction via regulating ERK5-mediated degradation of inducible cAMP early repressor. FASEB J. 2010. 24:4917–4928.27. Fleming I, Bauersachs J, Fisslthaler B, Busse R. Ca2+-independent activation of the endothelial nitric oxide synthase in response to tyrosine phosphatase inhibitors and fluid shear stress. Circ Res. 1998. 82:686–695.28. Woo CH, Massett MP, Shishido T, Itoh S, Ding B, McClain C, Che W, Vulapalli SR, Yan C, Abe J. ERK5 activation inhi bits inflammatory responses via peroxisome proliferator-ac ti vated receptor delta (PPARdelta) stimulation. J Biol Chem. 2006. 281:32164–32174.29. Min JH, Kim DK, Kwak HH, Kim GC, Lee SE, Kim IR, Kim CH, Park BS. Chios gum mastic induces cell cycle arrest and apoptosis in human osteosarcoma cells. Korean J Anat. 2009. 42:245–256.30. Brooks CL, Gu W. p53 regulation by ubiquitin. FEBS Lett. 2011. 585:2803–2809.31. Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003. 11:577–590.32. Carter S, Bischof O, Dejean A, Vousden KH. C-terminal modifications regulate MDM2 dissociation and nuclear export of p53. Nat Cell Biol. 2007. 9:428–435.33. Li L, Tatake RJ, Natarajan K, Taba Y, Garin G, Tai C, Leung E, Surapisitchat J, Yoshizumi M, Yan C, Abe J, Berk BC. Fluid shear stress inhibits TNF-mediated JNK activation via MEK5-BMK1 in endothelial cells. Biochem Biophys Res Commun. 2008. 370:159–163.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- PIG3 Regulates p53 Stability by Suppressing Its MDM2-Mediated Ubiquitination
- The Pharmacological Inhibition of ERK5 Enhances Apoptosis in Acute Myeloid Leukemia Cells
- p53 signaling is involved in leptin-induced growth of hepatic and breast cancer cells
- p53-mediated Inhibitory Mechanism on HIV-1 Tat is Likely to be Associated with Tat-Phosphorylation
- The Antitumor Effect of C-terminus of Hsp70-Interacting Protein via Degradation of c-Met in Small Cell Lung Cancer