J Korean Surg Soc.
2011 Oct;81(4):263-270. 10.4174/jkss.2011.81.4.263.
Significance of CD133 as a cancer stem cell markers focusing on the tumorigenicity of pancreatic cancer cell lines
- Affiliations
-
- 1Department of Health Science and Technology, Samsung Advanced Institute of Health Science and Technology, Sungkyunkwan University Graduate School, Seoul, Korea. dw7722.choi@samsung.com
- 2Samsung Biomedical Research Institute, Seoul, Korea.
- 3Department of Surgery, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea.
- 4Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Surgery, Kwandong University College of Medicine Myongi Hospital, Goyang, Korea.
- KMID: 1445747
- DOI: http://doi.org/10.4174/jkss.2011.81.4.263
Abstract
- PURPOSE
The cancer stem cell hypothesis states that the capacity of a cancer to grow and propagate is dependent on a small subset of cells. To determine the significances of the cancer stem cell markers CD133, CD44, and CD24 using a comparative analysis with a focus on tumorigenicity.
METHODS
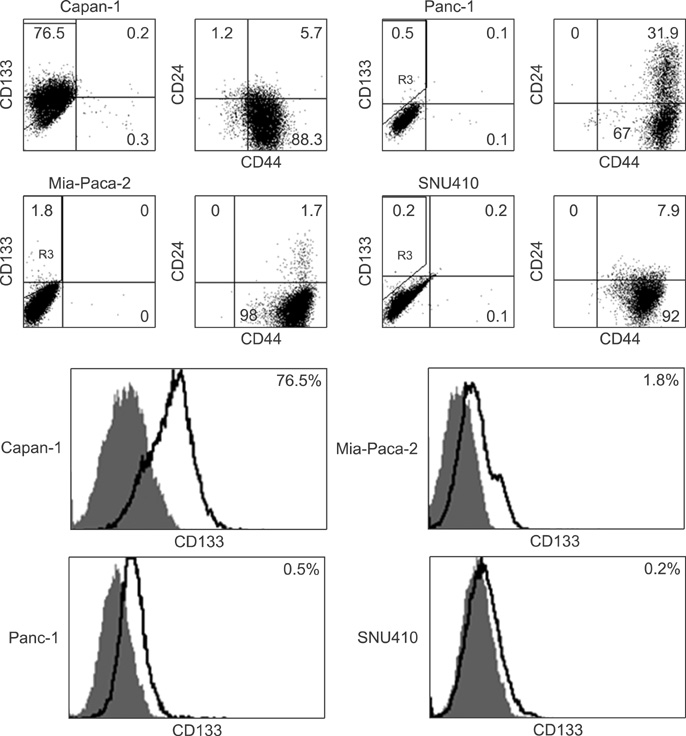
Four pancreatic cancer cell lines, Capan-1, Mia-PACA-2, Panc-1, and SNU-410 were analyzed for the expressions of CD133, CD44, and CD24 by flow cytometry. The tumorigenicity was compared using tumor volumes and numbers of tumors formed/numbers of injection in nonobese diabetic severe combined deficiency mice. Fluorescence-activated cell sorting (FACS) analysis was used to confirm that xenograft explants originated from human pancreatic cancer cells.
RESULTS
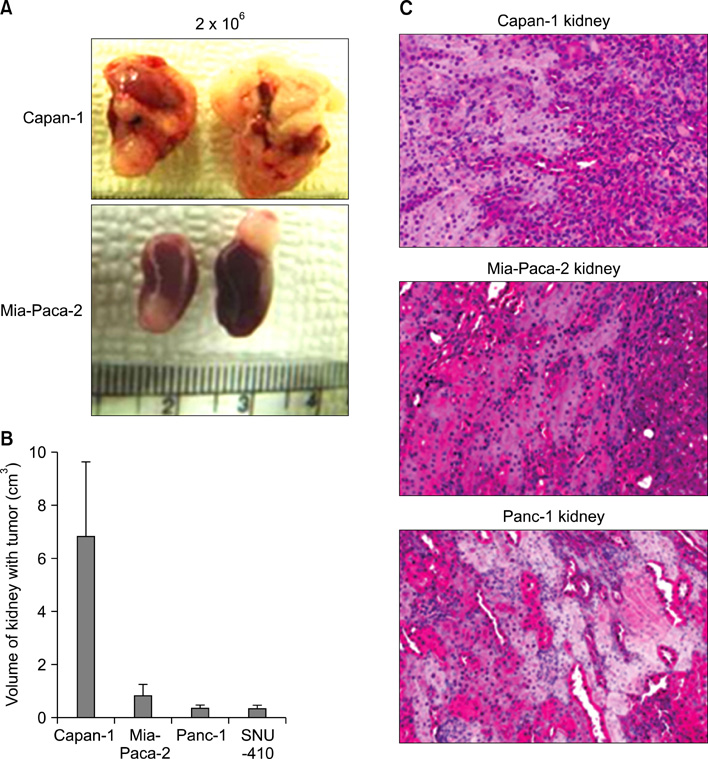
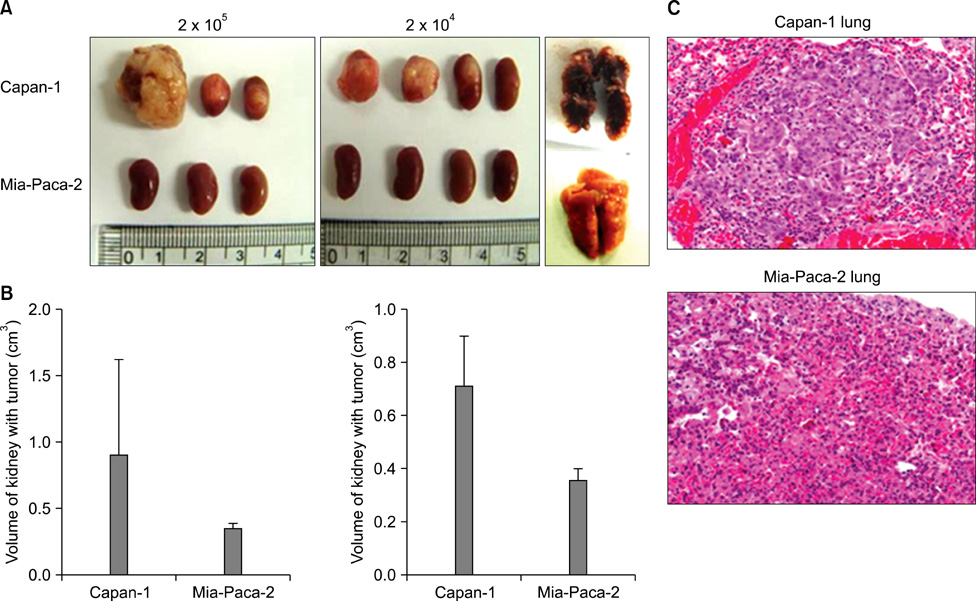
CD133 was positive in only Capan-1, CD44 positive in all, CD24 partially positive in Panc-1. After injecting 2 x 10(6) cells, all mice administered Capan-1 or Mia-Paca-2 developed tumors, 3 of 5 administered Panc-1 developed tumors, but no mouse administered SNU-410 developed any tumors. The volumes of Capan-1 tumors were seven times larger than those of Mia-Paca-2 tumors. When 2 x 10(5) or 2 x 10(4) of Capan-1 or Mia-Paca-2 was injected, tumors developed in all Capan-1 treated mice, but not in Mia-Paca-2 treated mice. Furthermore, xenograft explants of Capan-1 expressed CD133+CD44+ and Capan-1 injected mice developed lung metastasis. FACS analysis showed that xenograft explants originated from human pancreatic cancer cell lines.
CONCLUSION
CD133 positive cells have higher tumorigenic and metastatic potential than CD44 and CD24 positive cells, which suggests that CD133 might be a meaningful cell surface marker of pancreatic cancer stem cells.
MeSH Terms
Figure
Reference
-
1. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001. 414:105–111.2. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003. 100:3983–3988.3. Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997. 3:730–737.4. Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004. 64:7011–7021.5. Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser , et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003. 100:15178–15183.6. Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994. 367:645–648.7. Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006. 25:1696–1708.8. Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004. 432:396–401.9. Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci U S A. 2006. 103:11154–11159.10. Bissell MJ, Labarge MA. Context, tissue plasticity, and cancer: are tumor stem cells also regulated by the microenvironment? Cancer Cell. 2005. 7:17–23.11. McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999. 5:1410–1412.12. Lee CJ, Dosch J, Simeone DM. Pancreatic cancer stem cells. J Clin Oncol. 2008. 26:2806–2812.13. Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006. 12:1167–1174.14. Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006. 442:818–822.15. Lee SM, Buchler T, Joseph T, Lai C. Bilateral eardrum perforation after long-term treatment with erlotinib. J Clin Oncol. 2008. 26:2582–2584.16. Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003. 4:33–45.17. Weichert W, Denkert C, Burkhardt M, Gansukh T, Bellach J, Altevogt P, et al. Cytoplasmic CD24 expression in colorectal cancer independently correlates with shortened patient survival. Clin Cancer Res. 2005. 11:6574–6581.18. Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007. 67:1030–1037.19. O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007. 445:106–110.20. Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007. 445:111–115.21. Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007. 1:313–323.22. Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV, Varticovski L. Brca1 breast tumors contain distinct CD44+/CD24= and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008. 10:R10.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CD44 and CD133 as Cancer Stem Cell Markers for Gastric Cancer
- Identification of new biomarkers of hepatic cancer stem cells through proteomic profiling
- Effect of 5-FU and MTX on the Expression of Drug-resistance Related Cancer Stem Cell Markers in Non-small Cell Lung Cancer Cells
- Expression of CD133, CD44, CK7, and OCT4 in Animal Cancers
- Relationship between Cancer Stem Cell Marker CD133 and Cancer Germline Antigen Genes in NCI-H292 Lung Cancer Cells