Korean J Physiol Pharmacol.
2013 Jun;17(3):237-243. 10.4196/kjpp.2013.17.3.237.
Cytotoxic Activity and Quantitative Structure Activity Relationships of Arylpropyl Sulfonamides
- Affiliations
-
- 1College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. chaeukim@cau.ac.kr
- KMID: 1429304
- DOI: http://doi.org/10.4196/kjpp.2013.17.3.237
Abstract
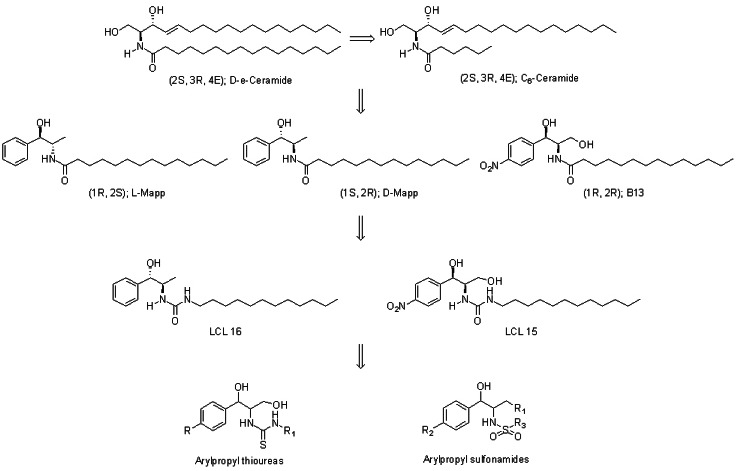
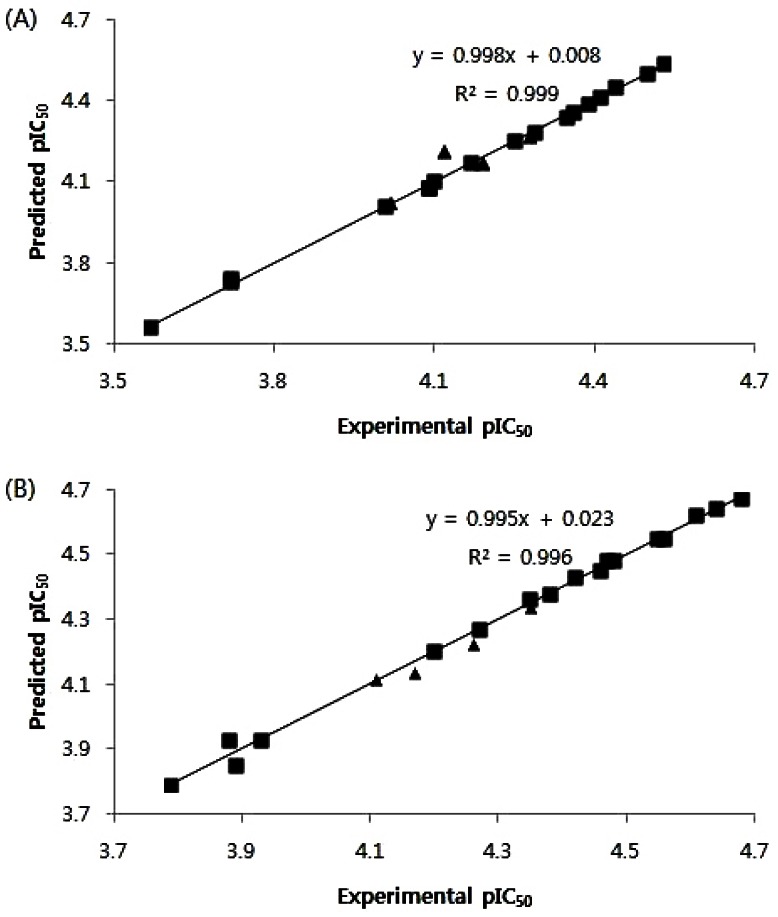
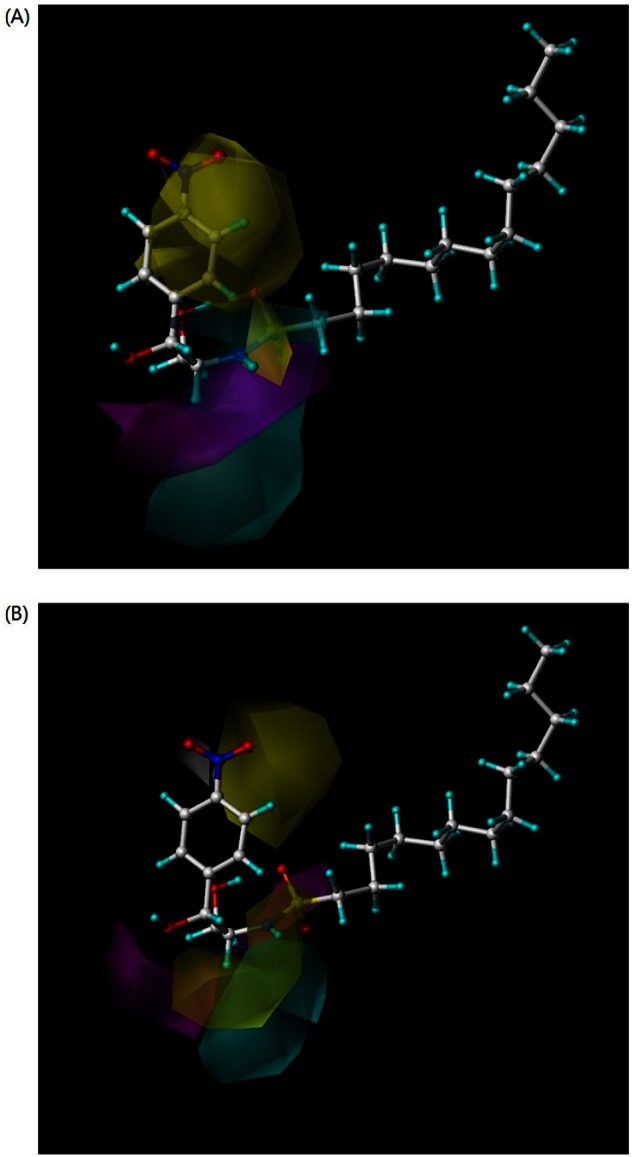
- B13 is a ceramide analogue and apoptosis inducer with potent cytotoxic activity. A series of arylpropyl sulfonamide analogues of B13 were evaluated for their cytotoxicity using MTT assays in prostate cancer PC-3 and leukemia HL-60 cell lines. Some compounds (4, 9, 13, 14, 15, and 20) showed stronger activities than B13 in both tumor cell lines, and compound (15) gave the most potent activity with IC50 values of 29.2 and 20.7 microM, for PC-3and HL-60 cells, respectively. Three-dimensional quantitative structure-activity relationship (3D-QSAR) analysis was performed to build highly reliable and predictive CoMSIA models with cross-validated q2 values of 0.816 and 0.702, respectively. Our results suggest that long alkyl chains and a 1R, 2R configuration of the propyl group are important for the cytotoxic activities of arylpropyl sulfonamides. Moreover, the introduction of small hydrophobic groups in the phenyl ring and sulfonamide group could increase biological activity.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Cytotoxicity and Structure-activity Relationships of Naphthyridine Derivatives in Human Cervical Cancer, Leukemia, and Prostate Cancer
Yu Jin Hwang, Mi Lyang Chung, Uy Dong Sohn, Chaeuk Im
Korean J Physiol Pharmacol. 2013;17(6):517-523. doi: 10.4196/kjpp.2013.17.6.517.
Reference
-
1. Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem J. 2004; 382:527–533. PMID: 15144238.
Article2. Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO Rep. 2004; 5:777–782. PMID: 15289826.
Article3. Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta. 2002; 1585:114–125. PMID: 12531544.
Article4. Carpinteiro A, Dumitru C, Schenck M, Gulbins E. Ceramide-induced cell death in malignant cells. Cancer Lett. 2008; 264:1–10. PMID: 18353542.
Article5. Kim HJ, Song JY, Park HJ, Park HK, Yun DH, Chung JH. Naringin protects against rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells. Korean J Physiol Pharmacol. 2009; 13:281–285. PMID: 19885011.
Article6. Fillet M, Bentires-Alj M, Deregowski V, Greimers R, Gielen J, Piette J, Bours V, Merville MP. Mechanisms involved in exogenous C2- and C6-ceramide-induced cancer cell toxicity. Biochem Pharmacol. 2003; 65:1633–1642. PMID: 12754099.
Article7. López-Marure R, Gutiérrez G, Mendoza C, Ventura JL, Sánchez L, Reyes Maldonado E, Zentella A, Montaño LF. Ceramide promotes the death of human cervical tumor cells in the absence of biochemical and morphological markers of apoptosis. Biochem Biophys Res Commun. 2002; 293:1028–1036. PMID: 12051763.
Article8. Ogretmen B, Pettus BJ, Rossi MJ, Wood R, Usta J, Szulc Z, Bielawska A, Obeid LM, Hannun YA. Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in the A549 human lung adenocarcinoma cell line. Role for endogenous ceramide in mediating the action of exogenous ceramide. J Biol Chem. 2002; 277:12960–12969. PMID: 11815611.9. Bedia C, Triola G, Casas J, Llebaria A, Fabriàs G. Analogs of the dihydroceramide desaturase inhibitor GT11 modified at the amide function: synthesis and biological activities. Org Biomol Chem. 2005; 3:3707–3712. PMID: 16211106.
Article10. Gouazé V, Liu YY, Prickett CS, Yu JY, Giuliano AE, Cabot MC. Glucosylceramide synthase blockade down-regulates P-glycoprotein and resensitizes multidrug-resistant breast cancer cells to anticancer drugs. Cancer Res. 2005; 65:3861–3867. PMID: 15867385.
Article11. Granot T, Milhas D, Carpentier S, Dagan A, Ségui B, Gatt S, Levade T. Caspase-dependent and -independent cell death of Jurkat human leukemia cells induced by novel synthetic ceramide analogs. Leukemia. 2006; 20:392–399. PMID: 16397504.
Article12. Grijalvo S, Bedia C, Triola G, Casas J, Llebaria A, Teixidó J, Rabal O, Levade T, Delgado A, Fabriàs G. Design, synthesis and activity as acid ceramidase inhibitors of 2-oxooctanoyl and N-oleoylethanolamine analogues. Chem Phys Lipids. 2006; 144:69–84. PMID: 16942762.
Article13. Morales A, París R, Villanueva A, Llacuna L, García-Ruiz C, Fernández-Checa JC. Pharmacological inhibition or small interfering RNA targeting acid ceramidase sensitizes hepatoma cells to chemotherapy and reduces tumor growth in vivo. Oncogene. 2007; 26:905–916. PMID: 16862171.14. Dindo D, Dahm F, Szulc Z, Bielawska A, Obeid LM, Hannun YA, Graf R, Clavien PA. Cationic long-chain ceramide LCL-30 induces cell death by mitochondrial targeting in SW403 cells. Mol Cancer Ther. 2006; 5:1520–1529. PMID: 16818511.
Article15. Senkal CE, Ponnusamy S, Rossi MJ, Sundararaj K, Szulc Z, Bielawski J, Bielawska A, Meyer M, Cobanoglu B, Koybasi S, Sinha D, Day TA, Obeid LM, Hannun YA, Ogretmen B. Potent antitumor activity of a novel cationic pyridinium-ceramide alone or in combination with gemcitabine against human head and neck squamous cell carcinomas in vitro and in vivo. J Pharmacol Exp Ther. 2006; 317:1188–1199. PMID: 16510697.16. Granot T, Milhas D, Carpentier S, Dagan A, Ségui B, Gatt S, Levade T. Caspase-dependent and -independent cell death of Jurkat human leukemia cells induced by novel synthetic ceramide analogs. Leukemia. 2006; 20:392–399. PMID: 16397504.
Article17. Nam EJ, Lee HS, Lee YJ, Joo WS, Maeng S, Im HI, Park CW, Kim YS. Ceramide is involved in MPP+ -induced cytotoxicity in human neuroblastoma cells. Korean J Physiol Pharmacol. 2002; 6:281–286.18. Ahn EH, Schroeder JJ. Induction of apoptosis by sphingosine, sphinganine, and C(2)-ceramide in human colon cancer cells, but not by C(2)-dihydroceramide. Anticancer Res. 2010; 30:2881–2884. PMID: 20683027.19. Chun YJ, Lee S, Yang SA, Park S, Kim MY. Modulation of CYP3A4 expression by ceramide in human colon carcinoma HT-29 cells. Biochem Biophys Res Commun. 2002; 298:687–692. PMID: 12419308.
Article20. Macchia M, Barontini S, Bertini S, Di Bussolo V, Fogli S, Giovannetti E, Grossi E, Minutolo F, Danesi R. Design, synthesis, and characterization of the antitumor activity of novel ceramide analogues. J Med Chem. 2001; 44:3994–4000. PMID: 11689086.
Article21. Oh JE, So KS, Lim SJ, Kim MY. Induction of apoptotic cell death by a ceramide analog in PC-3 prostate cancer cells. Arch Pharm Res. 2006; 29:1140–1146. PMID: 17225464.
Article22. Szulc ZM, Mayroo N, Bai A, Bielawski J, Liu X, Norris JS, Hannun YA, Bielawska A. Novel analogs of D-e-MAPP and B13. Part 1: synthesis and evaluation as potential anticancer agents. Bioorg Med Chem. 2008; 16:1015–1031. PMID: 17869115.
Article23. Alphonse G, Bionda C, Aloy MT, Ardail D, Rousson R, Rodriguez-Lafrasse C. Overcoming resistance to gamma-rays in squamous carcinoma cells by poly-drug elevation of ceramide levels. Oncogene. 2004; 23:2703–2715. PMID: 15048093.24. Lépine S, Lakatos B, Courageot MP, Le Stunff H, Sulpice JC, Giraud F. Sphingosine contributes to glucocorticoid-induced apoptosis of thymocytes independently of the mitochondrial pathway. J Immunol. 2004; 173:3783–3790. PMID: 15356125.
Article25. Maupas-Schwalm F, Augé N, Robinet C, Cambus JP, Parsons SJ, Salvayre R, Nègre-Salvayre A. The sphingomyelin/ceramide pathway is involved in ERK1/2 phosphorylation, cell proliferation, and uPAR overexpression induced by tissue-type plasminogen activator. FASEB J. 2004; 18:1398–1400. PMID: 15231724.26. Rodriguez-Lafrasse C, Alphonse G, Aloy MT, Ardail D, Gerard JP, Louisot P, Rousson R. Increasing endogenous ceramide using inhibitors of sphingolipid metabolism maximizes ionizing radiation-reduced mitochondrial injury and apoptotic cell killing. Int J Cancer. 2002; 101:589–598. PMID: 12237902.27. Raisova M, Goltz G, Bektas M, Bielawska A, Riebeling C, Hossini AM, Eberle J, Hannun YA, Orfanos CE, Geilen CC. Bcl-2 overexpression prevents apoptosis induced by ceramidase inhibitors in malignant melanoma and HaCaT keratinocytes. FEBS Lett. 2002; 516:47–52. PMID: 11959101.
Article28. Samsel L, Zaidel G, Drumgoole HM, Jelovac D, Drachenberg C, Rhee JG, Brodie AM, Bielawska A, Smyth MJ. The ceramide analog, B13, induces apoptosis in prostate cancer cell lines and inhibits tumor growth in prostate cancer xenografts. Prostate. 2004; 58:382–393. PMID: 14968439.
Article29. Selzner M, Bielawska A, Morse MA, Rüdiger HA, Sindram D, Hannun YA, Clavien PA. Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 2001; 61:1233–1240. PMID: 11221856.30. Samsel L, Zaidel G, Drumgoole HM, Jelovac D, Drachenberg C, Rhee JG, Brodie AM, Bielawska A, Smyth MJ. The ceramide analog, B13, induces apoptosis in prostate cancer cell lines and inhibits tumor growth in prostate cancer xenografts. Prostate. 2004; 58:382–393. PMID: 14968439.
Article31. Holman DH, Turner LS, El-Zawahry A, Elojeimy S, Liu X, Bielawski J, Szulc ZM, Norris K, Zeidan YH, Hannun YA, Bielawska A, Norris JS. Lysosomotropic acid ceramidase inhibitor induces apoptosis in prostate cancer cells. Cancer Chemother Pharmacol. 2008; 61:231–242. PMID: 17429631.
Article32. Usta J, El Bawab S, Roddy P, Szulc ZM, Yusuf , Hannun A, Bielawska A. Structural requirements of ceramide and sphingosine based inhibitors of mitochondrial ceramidase. Biochemistry. 2001; 40:9657–9668. PMID: 11583166.
Article33. Boyd AE 3rd. Sulfonylurea receptors, ion channels, and fruit flies. Diabetes. 1988; 37:847–850. PMID: 2454858.
Article34. Maren TH. Relatons between structure and biological activity of sulfonamides. Annu Rev Pharmacol Toxicol. 1976; 16:309–327. PMID: 59572.35. Abbate F, Casini A, Owa T, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: E7070,a sulfonamide anticancer agent, potently inhibits cytosolic isozymes I and II, and transmembrane, tumor-associated isozyme IX. Bioorg Med Chem Lett. 2004; 14:217–223. PMID: 14684331.36. Rostom SA. Synthesis and in vitro antitumor evaluation of some indeno[1,2-c]pyrazol(in)es substituted with sulfonamide, sulfonylurea(-thiourea) pharmacophores, and some derived thiazole ring systems. Bioorg Med Chem. 2006; 14:6475–6485. PMID: 16806944.
Article37. Kim YJ, Kim EA, Sohn UD, Yim CB, Im C. Cytotoxic Activity and Structure Activity Relationship of Ceramide Analogues in Caki-2 and HL-60 Cells. Korean J Physiol Pharmacol. 2010; 14:441–447. PMID: 21311687.
Article38. Park SM. Synthesis and anti-cancer activity of alkylsulfone amide derivatives. 2002. Chung-Ang Graduate School Master Thesis.39. Park JG, Kramer BS, Steinberg SM, Carmichael J, Collins JM, Minna JD, Gazdar AF. Chemosensitivity testing of human colorectal carcinoma cell lines using a tetrazolium-based colorimetric assay. Cancer Res. 1987; 47:5875–5879. PMID: 3664487.40. SYBYL Molecular Modeling Software. St. Louis, USA: Tripos Inc;2012.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytotoxicities and Quantitative Structure Activity Relationships of B13 Sulfonamides in HT-29 and A549 Cells
- Cytotoxic Activity and Three-Dimensional Quantitative Structure Activity Relationship of 2-Aryl-1,8-naphthyridin-4-ones
- Quantitative Structure Activity Relationship between Diazabicyclo[4.2.0]octanes Derivatives and Nicotinic Acetylcholine Receptor Agonists
- Cytotoxicity and Structure-activity Relationships of Naphthyridine Derivatives in Human Cervical Cancer, Leukemia, and Prostate Cancer
- Cytotoxic Lactones from the Pericarps of Litsea japonica