Korean J Physiol Pharmacol.
2013 Dec;17(6):517-523. 10.4196/kjpp.2013.17.6.517.
Cytotoxicity and Structure-activity Relationships of Naphthyridine Derivatives in Human Cervical Cancer, Leukemia, and Prostate Cancer
- Affiliations
-
- 1College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. chaeukim@cau.ac.kr
- KMID: 2285479
- DOI: http://doi.org/10.4196/kjpp.2013.17.6.517
Abstract
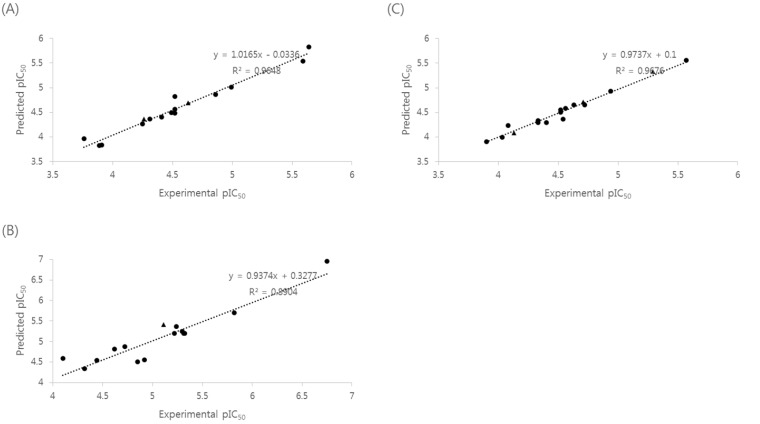
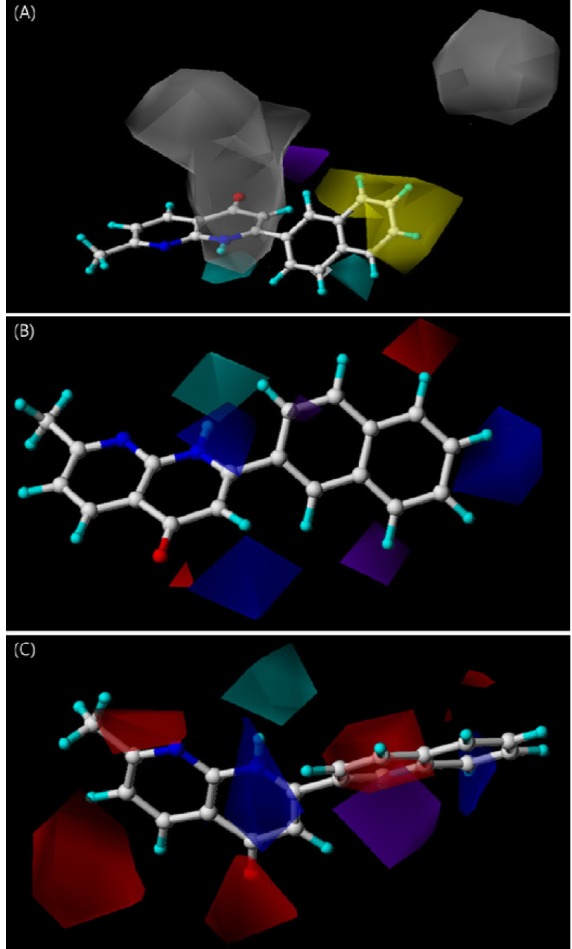
- Naphthyridine compounds are important, because they exhibit various biological activities including anticancer, antimicrobial, and anti-inflammatory activity. Some naphthyridines have antimitotic effects or demonstrate anticancer activity by inhibiting topoisomerase II. These compounds have been investigated as potential anticancer agents, and several compounds are now part of clinical trials. A series of naphthyridine derivatives were evaluated for their in vitro cytotoxic activities against human cervical cancer (HeLa), leukemia (HL-60), and prostate cancer (PC-3) cell lines using an MTT assay. Some compounds (14, 15, and 16) were more potent than colchicine against all three human cancer cell lines and compound (16) demonstrated potency with IC50 values of 0.7, 0.1, and 5.1 microM, respectively. Comparative molecular field analysis (CoMFA) and comparative molecular similarity indices analysis (CoMSIA) were used for quantitative structure-activity relationship (QSAR) molecular modeling of these compounds. We obtained accurate and predictive three-dimensional QSAR (3D-QSAR) models as indicated by the high PLS parameters of the HeLa (q2, 0.857; r2, 0.984; r2pred, 0.966), HL-60 (q2, 0.777; r2, 0.937; r2pred, 0.913), and PC-3 (q2, 0.702; r2, 0.983; r2pred, 0.974) cell lines. The 3D-QSAR contour maps suggested that the C-1 NH and C-4 carbonyl group of the naphthyridine ring and the C-2 naphthyl ring were important for cytotoxicity in all three human cancer cell lines.
Keyword
MeSH Terms
-
Antineoplastic Agents
Cell Line
Colchicine
DNA Topoisomerases, Type II
Humans*
Inhibitory Concentration 50
Leukemia*
Models, Molecular
Naphthyridines
Prostate*
Prostatic Neoplasms*
Quantitative Structure-Activity Relationship
Structure-Activity Relationship*
Uterine Cervical Neoplasms*
Antineoplastic Agents
Colchicine
DNA Topoisomerases, Type II
Naphthyridines
Figure
Cited by 1 articles
-
Immunotoxicological Effects of Aripiprazole:
In vivo andIn vitro Studies
Kwang-Soo Baek, Shinbyoung Ahn, Jaehwi Lee, Ji Hye Kim, Han Gyung Kim, Eunji Kim, Jun Ho Kim, Nak Yoon Sung, Sungjae Yang, Mi Seon Kim, Sungyoul Hong, Jong-Hoon Kim, Jae Youl Cho
Korean J Physiol Pharmacol. 2015;19(4):365-372. doi: 10.4196/kjpp.2015.19.4.365.
Reference
-
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008; 58:71–96. PMID: 18287387.
Article2. Hu WP, Yu HS, Chen YR, Tsai YM, Chen YK, Liao CC, Chang LS, Wang JJ. Synthesis and biological evaluation of thiobenzanilides as anticancer agents. Bioorg Med Chem. 2008; 16:5295–5302. PMID: 18359635.
Article3. Kemnitzer W, Jiang S, Wang Y, Kasibhatla S, Crogan-Grundy C, Bubenik M, Labrecque D, Denis R, Lamothe S, Attardo G, Gourdeau H, Tseng B, Drewe J, Cai SX. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based HTS assay. Part 5: modifications of the 2- and 3-positions. Bioorg Med Chem Lett. 2008; 18:603–607. PMID: 18077161.
Article4. Wordenmam L, Mitchison TJ. Dynamics of microtubule assembly in vivo. Mod Cell Biol. 1994; 13:287–301.5. Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004; 4:253–265. PMID: 15057285.
Article6. Xia Y, Yang ZY, Xia P, Bastow KF, Tachibana Y, Kuo SC, Hamel E, Hackl T, Lee KH. Antitumor agents. 181. Synthesis and biological evaluation of 6,7,2',3',4'-substituted-1,2,3,4-tetrahydro-2-phenyl-4-quinolones as a new class of antimitotic antitumor agents. J Med Chem. 1998; 41:1155–1162. PMID: 9544215.
Article7. Rowinsky EK, Donehower RC. The clinical pharmacology and use of antimicrotubule agents in cancer chemotherapeutics. Pharmacol Ther. 1991; 52:35–84. PMID: 1687171.
Article8. Verweij J, Clavel M, Chevalier B. Paclitaxel (Taxol) and docetaxel (Taxotere): not simply two of a kind. Ann Oncol. 1994; 5:495–505. PMID: 7918121.9. Shi Q, Chen K, Li L, Chang JJ, Autry C, Kozuka M, Konoshima T, Estes JR, Lin CM, Hamel E. Antitumor agents, 154. Cytotoxic and antimitotic flavonols from Polanisia dodecandra. J Nat Prod. 1995; 58:475–482. PMID: 7623025.
Article10. Manthey JA, Grohmann K, Guthrie N. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr Med Chem. 2001; 8:135–153. PMID: 11172671.
Article11. Hodek P, Trefil P, Stiborová M. Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem Biol Interact. 2002; 139:1–21. PMID: 11803026.
Article12. Manthey JA, Guthrie N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J Agric Food Chem. 2002; 50:5837–5843. PMID: 12358447.
Article13. Chan HY, Wang H, Leung LK. The red clover (Trifolium pratense) isoflavone biochanin A modulates the biotransformation pathways of 7,12-dimethylbenz[a]anthracene. Br J Nutr. 2003; 90:87–92. PMID: 12844379.14. Lee D, Bhat KP, Fong HH, Farnsworth NR, Pezzuto JM, Kinghorn AD. Aromatase inhibitors from Broussonetia papyrifera. J Nat Prod. 2001; 64:1286–1293. PMID: 11678652.
Article15. Pouget C, Fagnere C, Basly JP, Besson AE, Champavier Y, Habrioux G, Chulia AJ. Synthesis and aromatase inhibitory activity of flavanones. Pharm Res. 2002; 19:286–291. PMID: 11934235.16. Lai YY, Huang LJ, Lee KH, Xiao Z, Bastow KF, Yamori T, Kuo SC. Synthesis and biological relationships of 3',6-substituted 2-phenyl-4-quinolone-3-carboxylic acid derivatives as antimitotic agents. Bioorg Med Chem. 2005; 13:265–275. PMID: 15582470.
Article17. Li L, Wang HK, Kuo SC, Wu TS, Lednicer D, Lin CM, Hamel E, Lee KH. Antitumor agents. 150. 2',3',4',5',5,6,7-substituted 2-phenyl-4-quinolones and related compounds: their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J Med Chem. 1994; 37:1126–1135. PMID: 8164254.
Article18. Zhang SX, Bastow KF, Tachibana Y, Kuo SC, Hamel E, Mauger A, Narayanan VL, Lee KH. Antitumor agents. 196. Substituted 2-thienyl-1,8-naphthyridin-4-ones: their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J Med Chem. 1999; 42:4081–4087. PMID: 10514278.
Article19. Chen K, Kuo SC, Hsieh MC, Mauger A, Lin CM, Hamel E, Lee KH. Antitumor agents. 174. 2',3',4',5,6,7-Substituted 2-phenyl-1,8-naphthyridin-4-ones: their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J Med Chem. 1997; 40:2266–2275. PMID: 9216846.
Article20. Litvinov VP. Chemistry and biological activities of 1,8-naphthyridines. Russ Chem Rev. 2004; 73:637–670.
Article21. Kren V, Rezanka T. Sweet antibiotics - the role of glycosidic residues in antibiotic and antitumor activity and their randomization. FEMS Microbiol Rev. 2008; 32:858–889. PMID: 18647177.
Article22. Tsuzuki Y, Tomita K, Shibamori K, Sato Y, Kashimoto S, Chiba K. Synthesis and structure-activity relationships of novel 7-substituted 1,4-dihydro-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acids as antitumor agents. Part 2. J Med Chem. 2004; 47:2097–2109. PMID: 15056007.
Article23. Tomita K, Tsuzuki Y, Shibamori K, Tashima M, Kajikawa F, Sato Y, Kashimoto S, Chiba K, Hino K. Synthesis and structure-activity relationships of novel 7-substituted 1,4-dihydro-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acids as antitumor agents. Part 1. J Med Chem. 2002; 45:5564–5575. PMID: 12459024.
Article24. Srivastava SK, Jha A, Agarwal SK, Mukherjee R, Burman AC. Synthesis and structure-activity relationships of potent antitumor active quinoline and naphthyridine derivatives. Anticancer Agents Med Chem. 2007; 7:685–709. PMID: 18045063.
Article25. Tsuzuki Y, Tomita K, Sato Y, Kashimoto S, Chiba K. Synthesis and structure-activity relationships of 3-substituted 1,4-dihydro-4-oxo-1-(2-thiazolyl)-1,8-naphthyridines as novel antitumor agents. Bioorg Med Chem Lett. 2004; 14:3189–3193. PMID: 15149673.
Article26. Abbas JA, Stuart RK. Vosaroxin: a novel antineoplastic quinolone. Expert Opin Investig Drugs. 2012; 21:1223–1233.
Article27. Deady LW, Rogers ML, Zhuang L, Baguley BC, Denny WA. Synthesis and cytotoxic activity of carboxamide derivatives of benzo[b][1,6]naphthyridin-(5H)ones. Bioorg Med Chem. 2005; 13:1341–1355. PMID: 15670942.
Article28. Park JG, Kramer BS, Steinberg SM, Carmichael J, Collins JM, Minna JD, Gazdar AF. Chemosensitivity testing of human colorectal carcinoma cell lines using a tetrazolium-based colorimetric assay. Cancer Res. 1987; 47:5875–5879. PMID: 3664487.29. Manthey JA, Guthrie N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J Agric Food Chem. 2002; 50:5837–5843. PMID: 12358447.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytotoxic Activity and Quantitative Structure Activity Relationships of Arylpropyl Sulfonamides
- Diagnosis of Prostate Cancer
- The Diagnostic Value of Prostate-specific Antigen and the of Routine Laboratory Examination for Early Detection
- Cytotoxicities and Quantitative Structure Activity Relationships of B13 Sulfonamides in HT-29 and A549 Cells
- Expression of Survivin Correlated with Antiapoptosis in Benign Prostate Hyperplasia and Prostate Cancer