Korean J Physiol Pharmacol.
2011 Dec;15(6):423-429. 10.4196/kjpp.2011.15.6.423.
Cytotoxicities and Quantitative Structure Activity Relationships of B13 Sulfonamides in HT-29 and A549 Cells
- Affiliations
-
- 1College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. chaeukim@cau.ac.kr
- KMID: 2285430
- DOI: http://doi.org/10.4196/kjpp.2011.15.6.423
Abstract
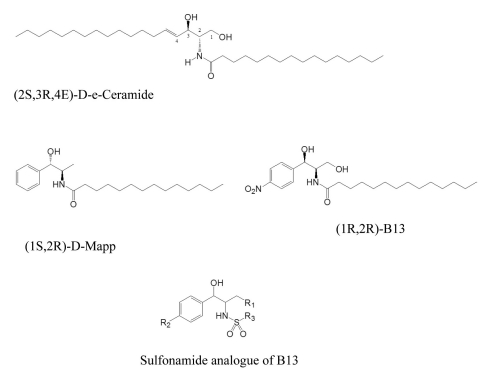
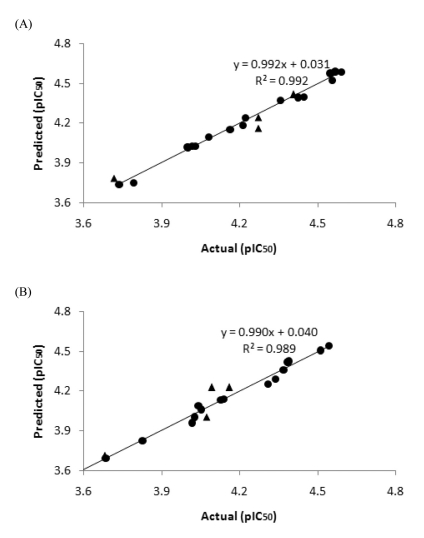
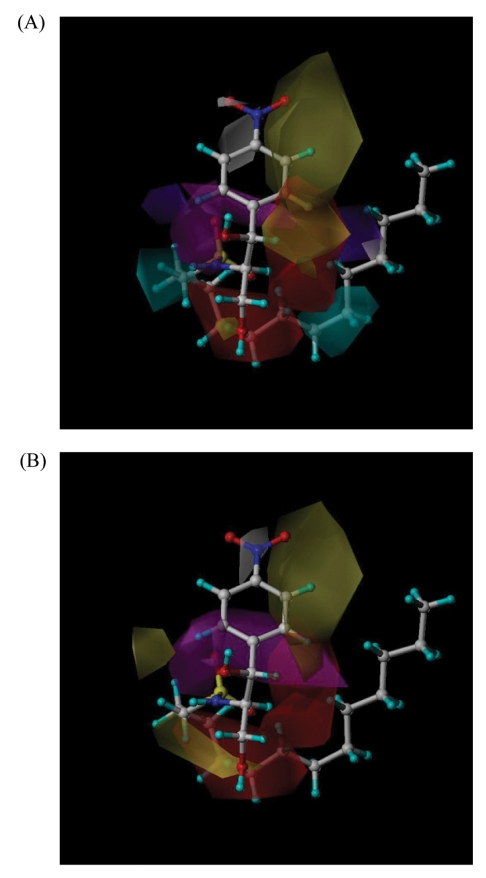
- B13 analogues are being considered as therapeutic agents for cancer cells, since B13 is a ceramide analogue and inhibits ceramidase to promote apoptosis in cancer cells. B13 sulfonamides are assumed to have biological activity similar to B13, since they are made by bioisosterically substituting the carboxyl moiety of B13 with sulfone group. Twenty B13 sulfonamides were evaluated for their in vitro cytotoxicities against human colon cancer HT-29 and lung cancer A549 cell lines using MTT assays. Replacement of the amide group with a sulfonamide group increased cytotoxicity in both cancer cell lines. The sulfonamides with long alkyl chains exhibited activities two to three times more potent than that of B13 and compound (15) had the most potent activity with IC50 values of 27 and 28.7microM for HT-29 and A549, respectively. The comparative molecular field analysis (CoMFA) and comparative molecular similarity indices analysis (CoMSIA) were used to carry out QSAR molecular modeling of these compounds. The predictive CoMSIA models for HT-29 and A549 gave cross-validated q2 values of 0.703 and 0.830, respectively. From graphical analysis of these models, we suppose that the stereochemistry of 1,3-propandiol is not important for activity and that introduction of a sulfonamide group and long alkyl chains into B13 can increase cytotoxicity.
Keyword
MeSH Terms
Figure
Reference
-
1. Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996; 274:1855–1859. PMID: 8943189.
Article2. Haimovitz-Friedman A, Kolesnick RN, Fuks Z. Ceramide signaling in apoptosis. Br Med Bull. 1997; 53:539–553. PMID: 9374036.
Article3. Fillet M, Bentires-Alj M, Deregowski V, Greimers R, Gielen J, Piette J, Bours V, Merville MP. Mechanisms involved in exogenous C2- and C6-ceramide-induced cancer cell toxicity. Biochem Pharmacol. 2003; 65:1633–1642. PMID: 12754099.
Article4. Kim HJ, Song JY, Park HJ, Park HK, Yun DH, Chung JH. Naringin Protects against Rotenone-induced Apoptosis in Human Neuroblastoma SH-SY5Y Cells. Korean J Physiol Pharmacol. 2009; 13:281–285. PMID: 19885011.
Article5. Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002; 277:25847–25850. PMID: 12011103.
Article6. Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003; 426:803–809. PMID: 14685229.
Article7. Lee DW, Park SY, Ryu JS, Kim SH, Im CU, Choi SH, Lee SE, Ko SK, Sohn UD. Relaxation effect of synthetic ceramide analogues in cat esophageal smooth muscle cells. Korean J Physiol Pharmacol. 2008; 12:137–142. PMID: 19967047.
Article8. Eto M, Bennouna J, Hunter OC, Hershberger PA, Kanto T, Johnson CS, Lotze MT, Amoscato AA. C16 ceramide accumulates following androgen ablation in LNCaP prostate cancer cells. Prostate. 2003; 57:66–79. PMID: 12886525.
Article9. Selzner M, Bielawska A, Morse MA, Rüdiger HA, Sindram D, Hannun YA, Clavien PA. Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 2001; 61:1233–1240. PMID: 11221856.10. Liu X, Elojeimy S, El-Zawahry AM, Holman DH, Bielawska A, Bielawski J, Rubinchik S, Guo GW, Dong JY, Keane T, Hannun YA, Tavassoli M, Norris JS. Modulation of ceramide metabolism enhances viral protein apoptin's cytotoxicity in prostate cancer. Mol Ther. 2006; 14:637–646. PMID: 16887394.
Article11. Senchenkov A, Litvak DA, Cabot MC. Targeting ceramide metabolism--a strategy for overcoming drug resistance. J Natl Cancer Inst. 2001; 93:347–357. PMID: 11238696.
Article12. Dindo D, Dahm F, Szulc Z, Bielawska A, Obeid LM, Hannun YA, Graf R, Clavien PA. Cationic long-chain ceramide LCL-30 induces cell death by mitochondrial targeting in SW403 cells. Mol Cancer Ther. 2006; 5:1520–1529. PMID: 16818511.
Article13. Senkal CE, Ponnusamy S, Rossi MJ, Sundararaj K, Szulc Z, Bielawski J, Bielawska A, Meyer M, Cobanoglu B, Koybasi S, Sinha D, Day TA, Obeid LM, Hannun YA, Ogretmen B. Potent antitumor activity of a novel cationic pyridinium-ceramide alone or in combination with gemcitabine against human head and neck squamous cell carcinomas in vitro and in vivo. J Pharmacol Exp Ther. 2006; 317:1188–1199. PMID: 16510697.14. Bedia C, Triola G, Casas J, Llebaria A, Fabriàs G. Analogs of the dihydroceramide desaturase inhibitor GT11 modified at the amide function: synthesis and biological activities. Org Biomol Chem. 2005; 3:3707–3712. PMID: 16211106.
Article15. Granot T, Milhas D, Carpentier S, Dagan A, Ségui B, Gatt S, Levade T. Caspase-dependent and -independent cell death of Jurkat human leukemia cells induced by novel synthetic ceramide analogs. Leukemia. 2006; 20:392–399. PMID: 16397504.
Article16. Morales A, París R, Villanueva A, Llacuna L, García-Ruiz C, Fernández-Checa JC. Pharmacological inhibition or small interfering RNA targeting acid ceramidase sensitizes hepatoma cells to chemotherapy and reduces tumor growth in vivo. Oncogene. 2007; 26:905–916. PMID: 16862171.17. Nam EJ, Lee HS, Lee YJ, Joo WS, Maeng SH, Im HI, Park CW, Kim YS. Ceramide is involved in MPP+-induced cytotoxicity in human neuroblastoma cells. Korean J Physiol Pharmacol. 2002; 6:281–286.18. Raisova M, Goltz G, Bektas M, Bielawska A, Riebeling C, Hossini AM, Eberle J, Hannun YA, Orfanos CE, Geilen CC. Bcl-2 overexpression prevents apoptosis induced by ceramidase inhibitors in malignant melanoma and HaCaT keratinocytes. FEBS Lett. 2002; 516:47–52. PMID: 11959101.
Article19. Selzner M, Bielawska A, Morse MA, Rüdiger HA, Sindram D, Hannun YA, Clavien PA. Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 2001; 61:1233–1240. PMID: 11221856.20. Alphonse G, Bionda C, Aloy MT, Ardail D, Rousson R, Rodriguez-Lafrasse C. Overcoming resistance to gamma-rays in squamous carcinoma cells by poly-drug elevation of ceramide levels. Oncogene. 2004; 23:2703–2715. PMID: 15048093.21. Lépine S, Lakatos B, Courageot MP, Le Stunff H, Sulpice JC, Giraud F. Sphingosine contributes to glucocorticoid-induced apoptosis of thymocytes independently of the mitochondrial pathway. J Immunol. 2004; 173:3783–3790. PMID: 15356125.
Article22. Bielawska A, Greenberg MS, Perry D, Jayadev S, Shayman JA, McKay C, Hannun YA. (1S,2R)-D-erythro-2-(N-myristoylamino)-1-phenyl-1-propanol as an inhibitor of ceramidase. J Biol Chem. 1996; 271:12646–12654. PMID: 8647877.
Article23. Samsel L, Zaidel G, Drumgoole HM, Jelovac D, Drachenberg C, Rhee JG, Brodie AM, Bielawska A, Smyth MJ. The ceramide analog, B13, induces apoptosis in prostate cancer cell lines and inhibits tumor growth in prostate cancer xenografts. Prostate. 2004; 58:382–393. PMID: 14968439.
Article24. Holman DH, Turner LS, El-Zawahry A, Elojeimy S, Liu X, Bielawski J, Szulc ZM, Norris K, Zeidan YH, Hannun YA, Bielawska A, Norris JS. Lysosomotropic acid ceramidase inhibitor induces apoptosis in prostate cancer cells. Cancer Chemother Pharmacol. 2008; 61:231–242. PMID: 17429631.
Article25. Usta J, El Bawab S, Roddy P, Szulc ZM, Yusuf , Hannun A, Bielawska A. Structural requirements of ceramide and sphingosine based inhibitors of mitochondrial ceramidase. Biochemistry. 2001; 40:9657–9668. PMID: 11583166.
Article26. Boyd AE 3rd. Sulfonylurea receptors, ion channels, and fruit flies. Diabetes. 1988; 37:847–850. PMID: 2454858.
Article27. Maren TH. Relatons between structure and biological activity of sulfonamides. Annu Rev Pharmacol Toxicol. 1976; 16:309–327. PMID: 59572.28. Drews J. Drug discovery: a historical perspective. Science. 2000; 287:1960–1964. PMID: 10720314.
Article29. Abbate F, Casini A, Owa T, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: E7070, a sulfonamide anticancer agent, potently inhibits cytosolic isozymes I and II, and transmembrane, tumor-associated isozyme IX. Bioorg Med Chem Lett. 2004; 14:217–223. PMID: 14684331.
Article30. Ghorab MM, Noaman E, Ismail MM, Heiba HI, Ammar YA, Sayed MY. Novel antitumor and radioprotective sulfonamides containing pyrrolo [2,3-d]pyrimidines. Arzneimittelforschung. 2006; 56:405–413. PMID: 16889123.31. Rostom SA. Synthesis and in vitro antitumor evaluation of some indeno[1,2-c]pyrazol(in)es substituted with sulfonamide, sulfonylurea(-thiourea) pharmacophores, and some derived thiazole ring systems. Bioorg Med Chem. 2006; 14:6475–6485. PMID: 16806944.
Article32. Supuran CT, Casini A, Mastrolorenzo A, Scozzafava A. COX-2 selective inhibitors, carbonic anhydrase inhibition and anticancer properties of sulfonamides belonging to this class of pharmacological agents. Mini Rev Med Chem. 2004; 4:625–632. PMID: 15279596.
Article33. Kim YJ, Kim EA, Sohn UD, Yim CB, Im C. Cytotoxic activity and structure activity relationship of ceramide analogues in Caki-2 and HL-60 cells. Korean J Physiol Pharmacol. 2010; 14:441–447. PMID: 21311687.
Article34. Park JG, Kramer BS, Steinberg SM, Carmichael J, Collins JM, Minna JD, Gazdar AF. Chemosensitivity testing of human colorectal carcinoma cell lines using a tetrazolium-based colorimetric assay. Cancer Res. 1987; 47:5875–5879. PMID: 3664487.35. SYBYL Molecular Modeling Software. 2010. USA: Tripos Inc., St. Louis.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytotoxic Activity and Quantitative Structure Activity Relationships of Arylpropyl Sulfonamides
- Cytotoxic Activity and Structure Activity Relationship of Ceramide Analogues in Caki-2 and HL-60 Cells
- Cytotoxic Activity and Three-Dimensional Quantitative Structure Activity Relationship of 2-Aryl-1,8-naphthyridin-4-ones
- Cholramphenicol and Sulfonamides
- Antimetastatic effect of fucoidan against non-small cell lung cancer by suppressing non-receptor tyrosine kinase and extracellular signalrelated kinase pathway