J Vet Sci.
2012 Sep;13(3):235-244. 10.4142/jvs.2012.13.3.235.
Activation of Akt/protein kinase B mediates the protective effects of mechanical stretching against myocardial ischemia-reperfusion injury
- Affiliations
-
- 1Department of Public Health, Chengdu Medical College, Chengdu, Sichuan 610083, China.
- 2Department of Pharmacology, College of Medicine, Chungbuk National University, Cheongju 361-763, Korea. kch@chungbuk.ac.kr
- 3Department of Thoracic and Cardiovascular Surgery, College of Medicine, Chungbuk National University, Cheongju 361-763, Korea. ksw713@chungbuk.ac.kr
- KMID: 1389762
- DOI: http://doi.org/10.4142/jvs.2012.13.3.235
Abstract
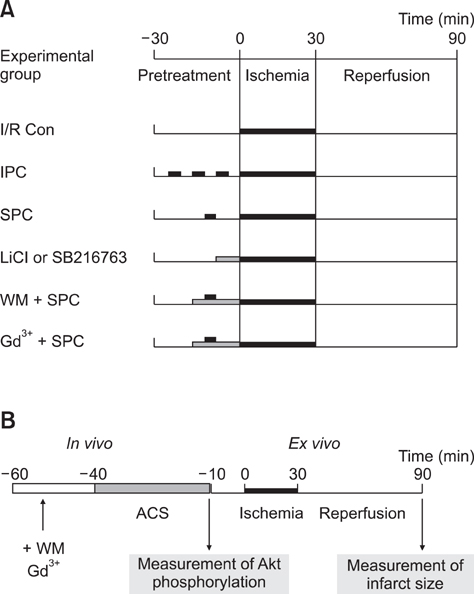
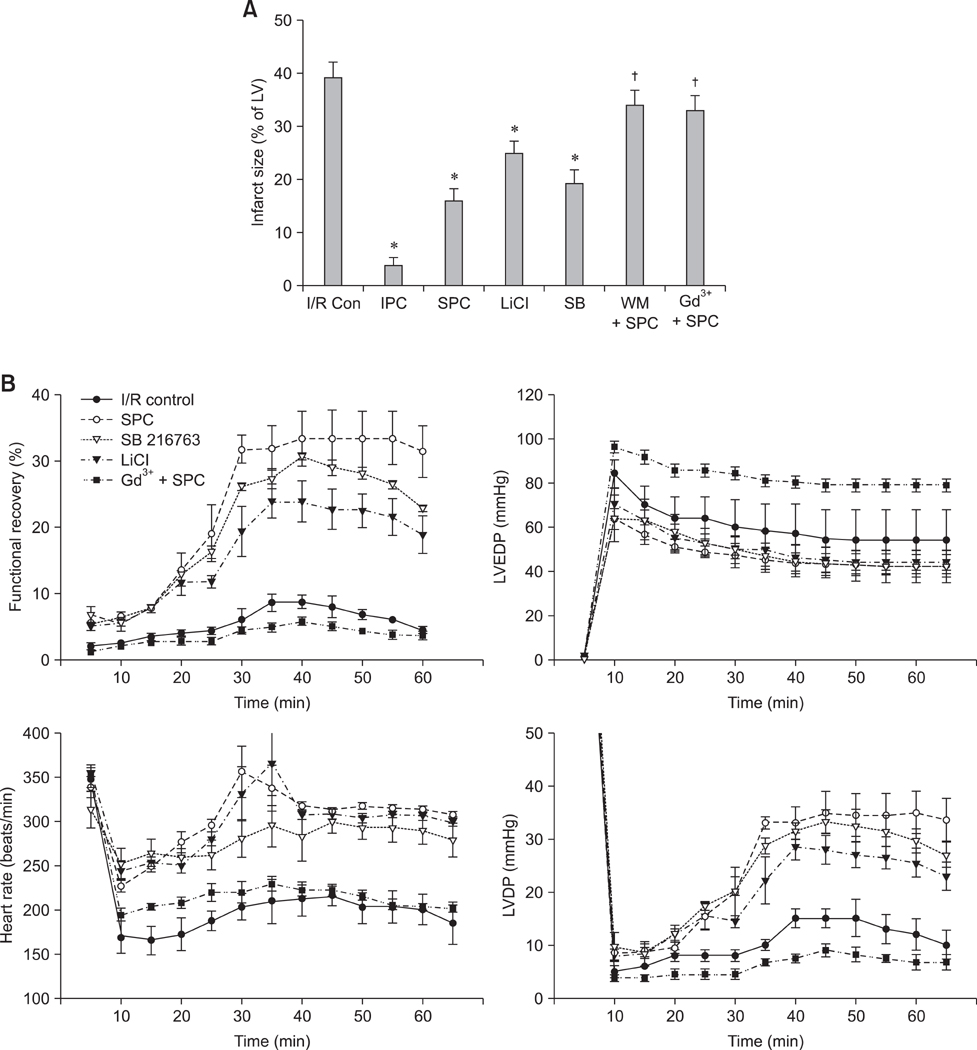
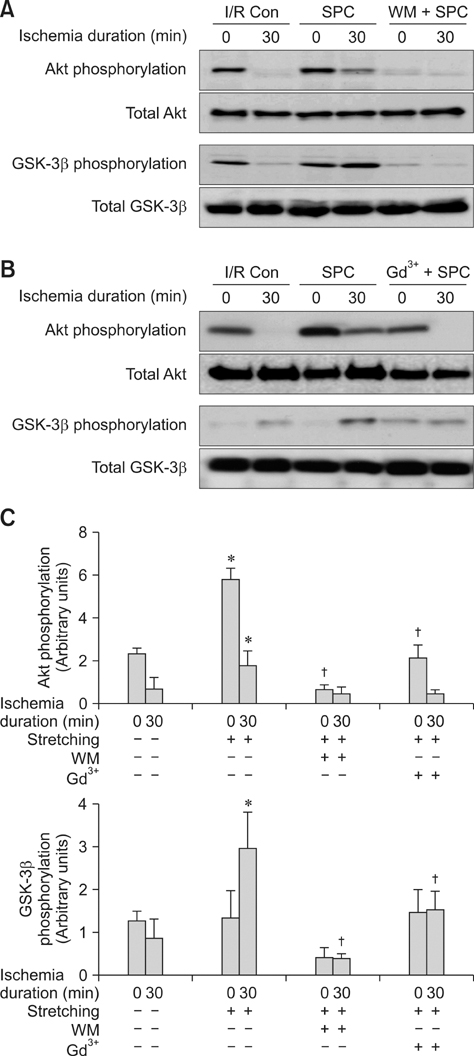
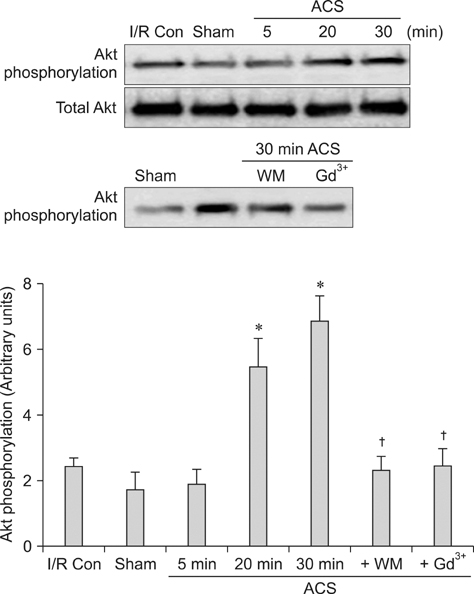
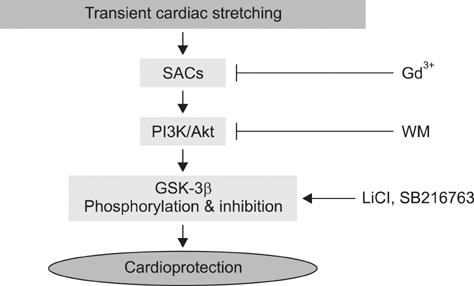
- Akt/protein kinase B is a well-known cell survival factor and activated by many stimuli including mechanical stretching. Therefore, we evaluated the cardioprotective effect of a brief mechanical stretching of rat hearts and determined whether activation of Akt through phosphatidylinositol 3-kinase (PI3K) is involved in stretch-induced cardioprotection (SIC). Stretch preconditioning reduced infarct size and improved post-ischemic cardiac function compared to the control group. Phosphorylation of Akt and its downstream substrate, GSK-3beta, was increased by mechanical stretching and completely blocked by wortmannin, a PI3K inhibitor. Treatment with lithium or SB216763 (GSK-3beta inhibitors) before ischemia induction mimicked the protective effects of SIC on rat heart. Gadolinium (Gd3+), a blocker of stretch-activated ion channels (SACs), inhibited the stretch-induced phosphorylation of Akt and GSK-3beta. Furthermore, SIC was abrogated by wortmannin and Gd3+. In vivo stretching induced by an aorto-caval shunt increased Akt phosphorylation and reduced myocardial infarction; these effects were diminished by wortmannin and Gd3+ pretreatment. Our results showed that mechanical stretching can provide cardioprotection against ischemia-reperfusion injury. Additionally, the activation of Akt, which might be regulated by SACs and the PI3K pathway, plays an important role in SIC.
MeSH Terms
-
Androstadienes/pharmacology
Animals
Gadolinium/pharmacology
Glycogen Synthase Kinase 3/*metabolism
Indoles/pharmacology
*Ischemic Preconditioning, Myocardial
Lithium/pharmacology
Male
Maleimides/pharmacology
Myocardial Reperfusion Injury/enzymology/physiopathology/*prevention & control
Phosphatidylinositol 3-Kinase/*antagonists & inhibitors/metabolism
Phosphorylation
Proto-Oncogene Proteins c-akt/*metabolism
Random Allocation
Rats
Rats, Sprague-Dawley
Specific Pathogen-Free Organisms
Figure
Reference
-
1. Aikawa R, Nawano M, Gu Y, Katagiri H, Asano T, Zhu W, Nagai R, Komuro I. Insulin prevents cardiomyocytes from oxidative stress-induced apoptosis through activation of PI3 kinase/Akt. Circulation. 2000. 102:2873–2879.
Article2. Badorff C, Ruetten H, Mueller S, Stahmer M, Gehring D, Jung F, Ihling C, Zeiher AM, Dimmeler S. Fas receptor signaling inhibits glycogen synthase kinase 3β and induces cardiac hypertrophy following pressure overload. J Clin Invest. 2002. 109:373–381.
Article3. Baines CP, Wang L, Cohen MV, Downey JM. Myocardial protection by insulin is dependent on phospatidylinositol 3-kinase but not protein kinase C or KATP channels in the isolated rabbit heart. Basic Res Cardiol. 1999. 94:188–198.
Article4. Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998. 282:1318–1321.
Article5. Das DK, Maulik N. Cardiac genomic response following preconditioning stimulus. Cardiovasc Res. 2006. 70:254–263.
Article6. Force T, Michael A, Kilter H, Haq S. Stretch-activated pathways and left ventricular remodeling. J Card Fail. 2002. 8:6 Suppl. S351–S358.
Article7. Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997. 88:435–437.
Article8. Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000. 101:660–667.
Article9. Gysembergh A, Margonari H, Loufoua J, Ovize A, André-Fouët X, Minaire Y, Ovize M. Stretch-induced protection shares a common mechanism with ischemic preconditioning in rabbit heart. Am J Physiol Heart Circ Physiol. 1998. 274:H955–H964.10. Haq S, Choukroun G, Kang ZB, Ranu H, Matsui T, Rosenzweig A, Molkentin JD, Alessandrini A, Woodgett J, Hajjar R, Michael A, Force T. Glycogen synthase kinase-3β is a negative regulator of cardiomyocyte hypertrophy. J Cell Biol. 2000. 151:117–129.
Article11. Hardt SE, Sadoshima J. Glycogen synthase kinase-3β: a novel regulator of cardiac hypertrophy and development. Circ Res. 2002. 90:1055–1063.12. Hong SJ, Dawson TM, Dawson VL. Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signaling. Trends Pharmacol Sci. 2004. 25:259–264.
Article13. Huang CH, Wang JS, Chiang SC, Wang YY, Lai ST, Weng ZC. Brief pressure overload of the left ventricle preconditions rabbit myocardium against infarction. Ann Thorac Surg. 2004. 78:628–633.
Article14. Illes RW, Swoyer KD. Prospective, randomized clinical study of ischemic preconditioning as an adjunct to intermittent cold blood cardioplegia. Ann Thorac Surg. 1998. 65:748–753.
Article15. Izuishi K, Tsung A, Hossain MA, Fujiwara M, Wakabayashi H, Masaki T, Billiar TR, Maeta H. Ischemic preconditioning of the murine liver protects through the Akt kinase pathway. Hepatology. 2006. 44:573–580.
Article16. Jiao JD, Garg V, Yang B, Hu K. Novel functional role of heat shock protein 90 in ATP-sensitive K+ channel-mediated hypoxic preconditioning. Cardiovasc Res. 2008. 77:126–133.
Article17. Kaukoranta PK, Lepojärvi MPK, Ylitalo KV, Kiviluoma KT, Peuhkurinen KJ. Normothermic retrograde blood cardioplegia with or without preceding ischemic preconditioning. Ann Thorac Surg. 1997. 63:1268–1274.
Article18. Kim CH, Cho YS, Chun YS, Park JW, Kim MS. Early expression of myocardial HIF-1α in response to mechanical stresses: regulation by stretch-activated channels and the phosphatidylinositol 3-kinase signaling pathway. Circ Res. 2002. 90:E25–E33.19. Kim CH, Choi H, Chun YS, Kim GT, Park JW, Kim MS. Hyperbaric oxygenation pretreatment induces catalase and reduces infarct size in ischemic rat myocardium. Pflugers Arch. 2001. 442:519–525.
Article20. Kim YH, Kim CH, Kim GT, Kim IK, Park JW, Kim MS. Involvement of adenosine in cardioprotective effect of catecholamine preconditioning in ischemia-reperfused heart of rat. Korean J Physiol Pharmacol. 1998. 2:753–761.21. Lammerding J, Kamm RD, Lee RT. Mechanotransduction in cardiac myocytes. Ann N Y Acad Sci. 2004. 1015:53–70.
Article22. Li B, Setoguchi M, Wang X, Andreoli AM, Leri A, Malhotra A, Kajstura J, Anversa P. Insulin-like growth factor-1 attenuates the detrimental impact of nonocclusive coronary artery constriction on the heart. Circ Res. 1999. 84:1007–1019.
Article23. Ma XJ, Yin HJ, Guo CY, Jiang YR, Wang JS, Shi DZ. Ischemic postconditioning through percutaneous transluminal coronary angioplasty in pigs: roles of PI3K activation. Coron Artery Dis. 2012. 23:245–250.
Article24. Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral gene transfer of activated phosphatidylinositol 3'-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation. 1999. 100:2373–2379.
Article25. Mosca SM. Cardioprotective effects of stretch are mediated by activation of sarcolemmal, not mitochondrial, ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol. 2007. 293:H1007–H1012.
Article26. Nagaya N, Nishikimi T, Yoshihara F, Horio T, Morimoto A, Kangawa K. Cardiac adrenomedullin gene expression and peptide accumulation after acute myocardial infarction in rats. Am J Physiol Regul Integr Comp Physiol. 2000. 278:R1019–R1026.
Article27. Nakagawa C, Asayama J, Katamura M, Matoba S, Keira N, Kawahara A, Tsuruyama K, Tanaka T, Kobara M, Akashi K, Ohta B, Tatsumi T, Nakagawa M. Myocardial stretch induced by increased left ventricular diastolic pressure preconditions isolated perfused hearts of normotensive and spontaneously hypertensive rats. Basic Res Cardiol. 1997. 92:410–416.
Article28. Ovize M, Kloner RA, Przyklenk K. Stretch preconditions canine myocardium. Am J Physiol Heart Circ Physiol. 1994. 266:H137–H146.
Article29. Pan J, Fukuda K, Saito M, Matsuzaki J, Kodama H, Sano M, Takahashi T, Kato T, Ogawa S. Mechanical stretch activates the JAK/STAT pathway in rat cardiomyocytes. Circ Res. 1999. 84:1127–1136.
Article30. Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998. 273:19929–19932.
Article31. Przyklenk K, Darling CE, Dickson EW, Whittaker P. Cardioprotection 'outside the box'--the evolving paradigm of remote preconditioning. Basic Res Cardiol. 2003. 98:149–157.32. Romashkova JA, Makarov SS. NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999. 401:86–90.
Article33. Scheid MP, Woodgett JR. PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol. 2001. 2:760–768.
Article34. Skurk C, Izumiya Y, Maatz H, Razeghi P, Shiojima I, Sandri M, Sato K, Zeng L, Schiekofer S, Pimentel D, Lecker S, Taegtmeyer H, Goldberg AL, Walsh K. The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J Biol Chem. 2005. 280:20814–20823.
Article35. Smith SC Jr. Reducing the global burden of ischemic heart disease and stroke. A challenge for the cardiovascular community and the United Nations. Circulation. 2011. 124:278–279.
Article36. Tapuria N, Kumar Y, Habib MM, Abu Amara M, Seifalian AM, Davidson BR. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury--a review. J Surg Res. 2008. 150:304–330.
Article37. Tong H, Chen W, Steenbergen C, Murphy E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ Res. 2000. 87:309–315.
Article38. Vaage J, Valen G. Preconditioning and cardiac surgery. Ann Thorac Surg. 2003. 75:S709–S714.
Article39. Wei H, van der Heide RS. Ischemic preconditioning and heat shock activate Akt via a focal adhesion kinase-mediated pathway in Langendorff-perfused adult rat hearts. Am J Physiol Heart Circ Physiol. 2010. 298:H152–H157.
Article40. Zhou H, Li XM, Meinkoth J, Pittman RN. Akt regulates cell survival and apoptosis at a postmitochondrial level. J Cell Biol. 2000. 151:483–494.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective Effect of Sauchinone Against Regional Myocardial Ischemia/Reperfusion Injury: Inhibition of p38 MAPK and JNK Death Signaling Pathways
- Mechanisms involved in adenosine pharmacological preconditioning-induced cardioprotection
- Mechanical Stretch-Induced Protection against Myocardial Ischemia-Reperfusion Injury Involves AMP-Activated Protein Kinase
- The effects of hydrogen sulfide under sevoflurane administration against ischemia and reperfusion injury in isolated rat heart
- Effects of sevoflurane on tight junction protein expression and PKC-alpha translocation after pulmonary ischemia-reperfusion injury