Korean J Physiol Pharmacol.
2010 Feb;14(1):1-9. 10.4196/kjpp.2010.14.1.1.
Mechanical Stretch-Induced Protection against Myocardial Ischemia-Reperfusion Injury Involves AMP-Activated Protein Kinase
- Affiliations
-
- 1Institute of Biomedical and Pharmaceutical Technology (IBPT), Fuzhou University, Fuzhou, 350002, PR China.
- 2Department of Pharmacology, College of Medicine, BK21 Chungbuk Biomedical Science Center, School of Medicine, Chungbuk National University, Cheongju 361-763, Korea. kch@chungbuk.ac.kr
- 3Department of Pediatrics, College of Medicine, BK21 Chungbuk Biomedical Science Center, School of Medicine, Chungbuk National University, Cheongju 361-763, Korea.
- KMID: 1457694
- DOI: http://doi.org/10.4196/kjpp.2010.14.1.1
Abstract
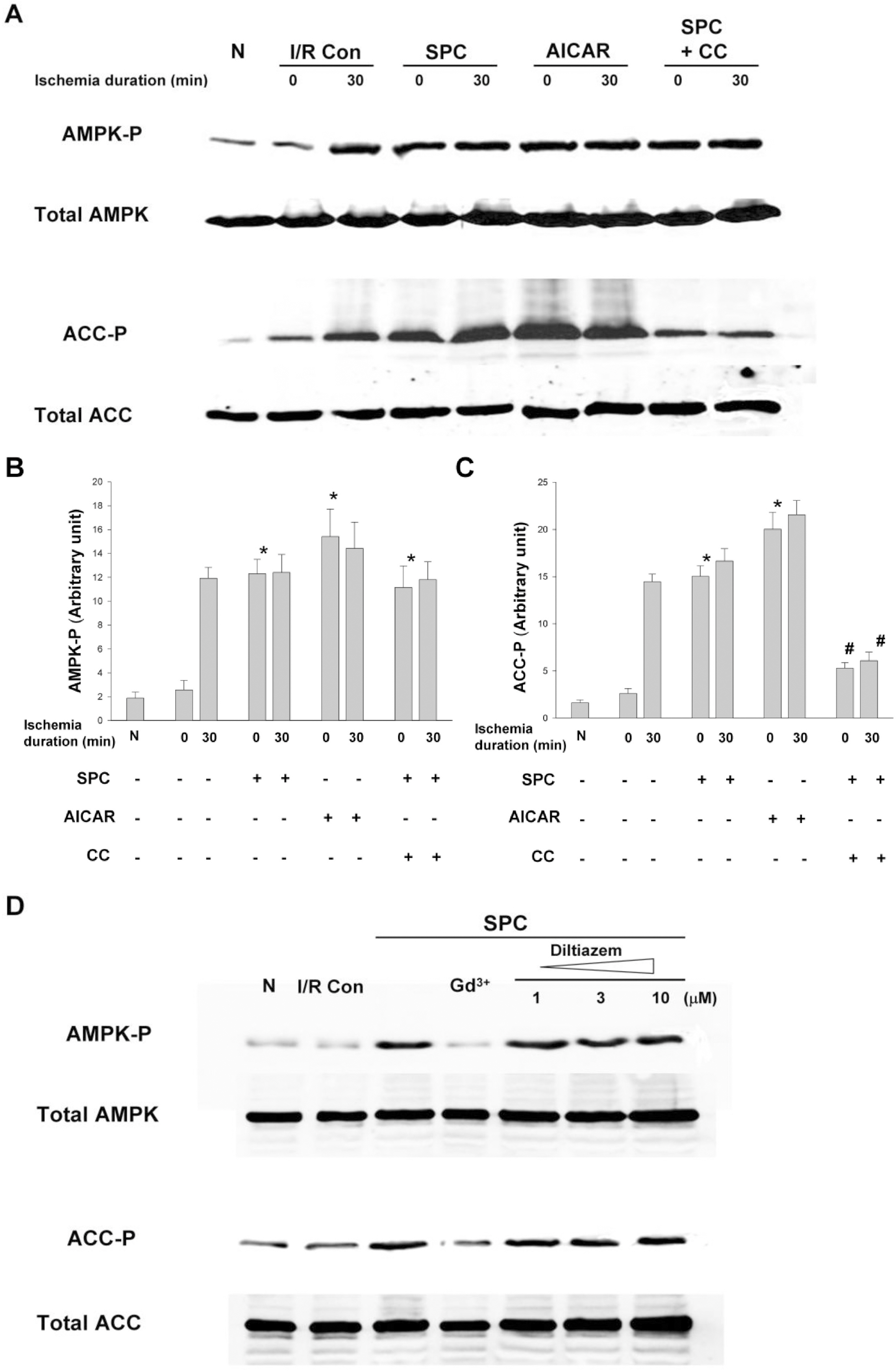
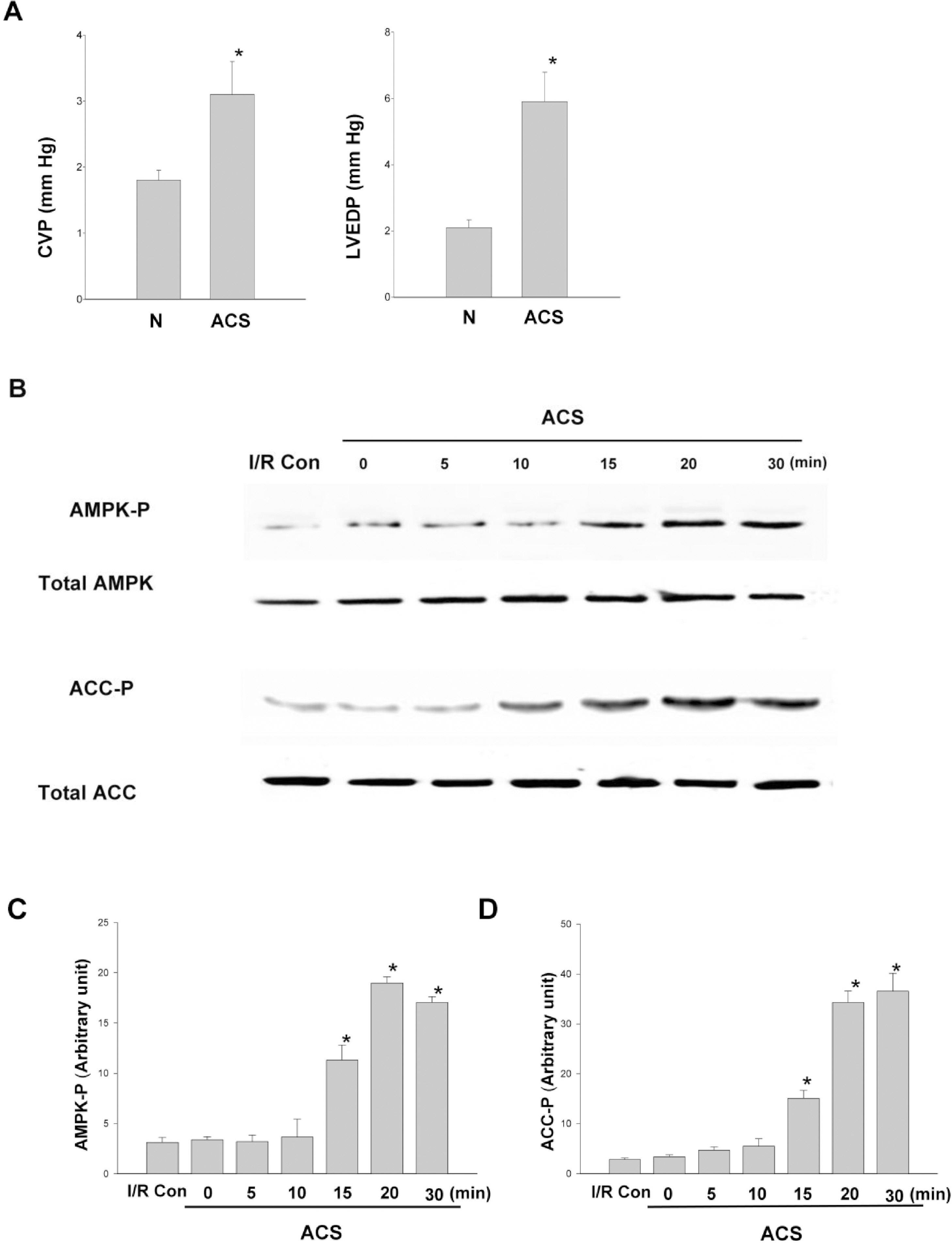
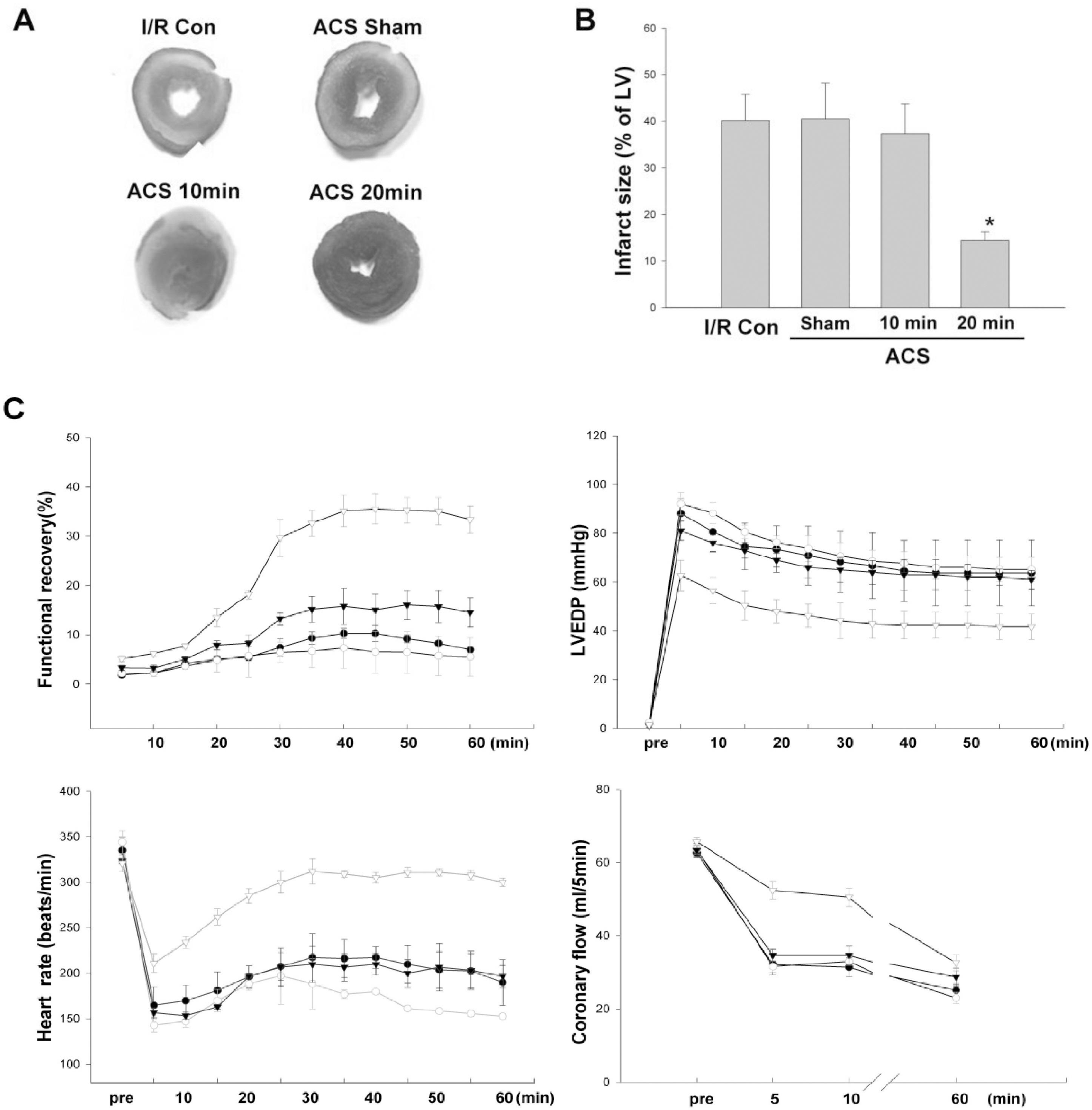
- AMP-activated protein kinase (AMPK) protects various tissues and cells from ischemic insults and is activated by many stimuli including mechanical stretch. Therefore, this study investigated if the activation of AMPK is involved in stretch-induced cardioprotection (SIC). Intraventricular balloon and aorto-caval shunt (ACS) were used to stretch rat hearts ex vivo and in vivo, respectively. Stretch preconditioning reduced myocardial infarct induced by ischemia-reperfusion (I/R) and improved post-ischemic functional recovery. Phosphorylation of AMPK and its downstream substrate, acetyl-CoA carboxylase (ACC) were increased by mechanical stretch and ACC phosphorylation was completely blocked by the AMPK inhibitor, Compound C. AMPK activator (AICAR) mimicked SIC. Gadolinium, a blocker of stretch-activated ion channels (SACs), inhibited the stretch-induced phosphorylation of AMPK and ACC, whereas diltiazem, a specific L-type calcium channel blocker, did not affect AMPK activation. Furthermore, SIC was abrogated by Compound C and gadolinium. The in vivo stretch induced by ACS increased AMPK activation and reduced myocardial infarct. These findings indicate that stretch preconditioning can induce the cardioprotection against I/R injury, and activation of AMPK plays an important role in SIC, which might be mediated by SACs.
MeSH Terms
Figure
Reference
-
References
1. Das DK, Maulik N. Cardiac genomic response following preconditioning stimulus. Cardiovasc Res. 2006; 70:254–263.
Article2. Shintani-Ishida K, Nakajima M, Uemura K, Yoshida K. Ischemic preconditioning protects cardiomyocytes against ischemic injury by inducing GRP78. Biochem Biophys Res Commun. 2006; 345:1600–1605.
Article3. Kim YH, Kim CH, Kim GT, Kim IK, Park JW, Kim MS. Involvement of adenosine in cardioprotective effect of catecholamine preconditioning in ischemia-reperfused heart of rat. Korean J Physiol Pharmacol. 1998; 2:753–761.4. Jiao JD, Garg V, Yang B, Hu K. Novel functional role of heat shock protein 90 in ATP-sensitive K+ channel-mediated hypoxic preconditioning. Cardiovasc Res. 2008; 77:126–133.5. Ovize M, Kloner RA, Przyklenk K. Stretch preconditions canine myocardium. Am J Physiol Heart Circ Physiol. 1994; 266:137–146.
Article6. Gysembergh A, Margonari H, Loufoua J, Ovize A, André-Fouët X, Minaire Y, Ovize M. Stretch-induced protection shares a common mechanism with ischemic preconditioning in rabbit heart. Am J Physiol Heart Circ Physiol. 1998; 274:955–964.7. Mosca SM. Cardioprotective effects of stretch are mediated by activation of sarcolemmal, not mitochondrial, ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol. 2007; 293:1007–1012.
Article8. Nakagawa C, Asayama J, Katamura M, Matoba S, Keira N, Kawahara A, Tsuruyama K, Tanaka T, Kobara M, Akashi K, Ohta B, Tatsumi T, Nakagawa M. Myocardial stretch induced by increased left ventricular diastolic pressure preconditions isolated perfused hearts of normotensive and spontaneously hypertensive rats. Basic Res Cardiol. 1997; 92:410–416.
Article9. Force T, Michael A, Kilter H, Haq S. Stretch-activated pathways and left ventricular remodeling. J Card Fail. 2002; 8:351–358.
Article10. Pan J, Fukuda K, Saito M, Matsuzaki J, Kodama H, Sano M, Takahashi T, Kato T, Ogawa S. Mechanical stretch activates the JAK/STAT pathway in rat cardiomyocytes. Circ Res. 1999; 84:1127–1236.
Article11. Wamel AJ, Ruwhof C, Valk-Kokshoom LE, Schrier PI, Laarse A. The role of angiotensin II, endothelin-1, and transforming growth factor as autocrine/paracrine mediators of stretch-induced cardiomyocyte hypertrophy. Mol Cell Biochem. 2001; 218:113–124.12. Smith MA, Moylan JS, Reid MB. Mechanical signal transduction in glucose transport of murine extensor digitorum longus (EDL) muscle. FASEB J. 2007; 21:94–98.
Article13. Rubin LJ, Magliola L, Feng X, Jones AW, Hale CC. Metabolic activation of AMP-kinase in vascular smooth muscle. J Appl Physiol. 2004; 10:1152–1175.14. Kahn BB, Alquier T, Carling D Hardie DG. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metabolism. 2005; 1:15–25.
Article15. Hardie DG, Salt IP, Hawley SA, Davies SP. AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochem J. 1999; 338:717–722.
Article16. Peralta C, Bartrons R, Serafin A, Blazquez C, Guzman M, Prats N, Xaus C, Cutillas B, Gelpí E, Roselló-Catafau J. Adenosine monophosphate activated protein kinase mediates the protective effects of ischemic preconditioning on hepatic ischemia-reperfusion injury in the rat. Hepatology. 2001; 34:1164–1173.17. Tian R, Musi N, D'Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001; 104:1664–1669.
Article18. Carling D. The AMP-activated protein kinase cascade – a unifying system for energy control. Trends Biochem Sci. 2004; 29:18–24.
Article19. Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001; 281:1340–1346.20. Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T, Araki E. Activation of AMP-activated protein kinase reduces hyper-glycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006; 55:120–127.
Article21. Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005; 25:9554–9575.
Article22. Russell I, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004; 114:495–503.
Article23. Sukhodub A, Jovanovi S, Qingyou DU, Budasl G, Clelland AK, Shen M, Sakamoto K, Tian R, Jovanović A. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K+ channels. J Cell Physiol. 2007; 210:224–236.24. Nishino Y, Miura T, Mikia T, Sakamoto J, Nakamura Y, Ikeda Y, Kobayashi H, Shimamoto K. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 up-regulation in the late phase of cardioprotection. Cardiovasc Res. 2004; 61:610–619.
Article25. Walker J, Jijon HB, Diaz H, Salehi P, Churchill T, Madsen KL. 5-Aminoimidazole-4-carboxamide riboside (AICAR) enhances GLUT2-dependent jejunal glucose transport: a possible role for AMPK. Biochem J. 2005; 385:485–491.
Article26. Gaskin FS, Kamada K, Yusof M, Korthuis RJ. 5-AMP-activated protein kinase activation prevents postischemic leukocyteen-dothelial cell adhesive interactions. Am J Physiol Heart Circ Physiol. 2007; 292:326–332.
Article27. Kim CH, Cho YS, Chun YS, Park JW, Kim MS. Early expression of myocardial HIF-1alpha in response to mechanical stresses: regulation by stretch-activated channels and the phosphatidylinositol 3-kinase signaling pathway. Circ Res. 2002; 90:E25–33.28. Nagaya N, Nishikimi T, Yoshihara F, Horio T, Morimoto A, Kangawa K. Cardiac adrenomedullin gene expression and peptide accumulation after acute myocardial infarction in rats. Am J Physiol Regul Integr Comp Physiol. 2000; 278:1019–1026.
Article29. Kim CH, Choi H, Chun YS, Kim GT, Park JW, Kim MS. Hyperbaric oxygenation pretreatment induces catalase and reduces infarct size in ischemic rat myocardium. Pflugers Arch. 2001; 442:519–525.
Article30. Sadoshima J, Takahashi T, Jahn L, Izumo S. Roles of mechano-sensitive ion channels, cytoskeleton, and contractile activity in stretch-induced immediate-early gene expression and hypertrophy of cardiac myocytes. Proc Natl Acad Sci USA. 1992; 89:9905–9909.
Article31. Biagi BA, Enyeart JJ. Gadolinium blocks low and high threshold calcium currents in pituitary cells. Am Physiol. 1990; 259:515–520.32. Huang CH, Wang JS, Chiang SC, Wang YY, Lai ST, Weng ZC. Brief pressure overload of the left ventricle preconditions rabbit myocardium against infarction. Ann Thorac Surg. 2004; 78:628–633.
Article33. Kudo N, Gillespie JG, Kung L, Witters LA, Schulz R, Clanachan AS, Lopaschuk GD. Characterization of 5-AMP activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta. 1996; 1301:67–75.34. Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003; 2:28–32.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Activation of Akt/protein kinase B mediates the protective effects of mechanical stretching against myocardial ischemia-reperfusion injury
- Activated Protein C Protects Myocardium Via Activation of Anti-apoptotic Pathways of Survival in Ischemia-reperfused Rat Heart
- Protective Effect of Sauchinone Against Regional Myocardial Ischemia/Reperfusion Injury: Inhibition of p38 MAPK and JNK Death Signaling Pathways
- The Role of the Adenosine Receptor Subtypes and Protein Kinase C in Ischemic Preconditioning in the in Vivo Cat Heart
- Morphine and remifentanil-induced cardioprotection: its experimental and clinical outcomes