J Vet Sci.
2012 Mar;13(1):43-48. 10.4142/jvs.2012.13.1.43.
Comparison of four diagnostic methods for detecting rabies viruses circulating in Korea
- Affiliations
-
- 1Animal, Plant and Fisheries Quarantine and Inspection Agency, Anyang 430-757, Korea. yangdk@korea.kr
- 2Gangwon-do Veterinary Laboratory Service, Chuncheon 200-822, Korea.
- 3Chungbuk Livestock and Veterinary Research Institute, Cheongwon 363-931, Korea.
- KMID: 1365002
- DOI: http://doi.org/10.4142/jvs.2012.13.1.43
Abstract
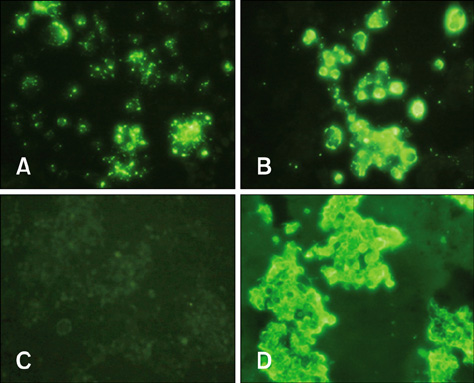
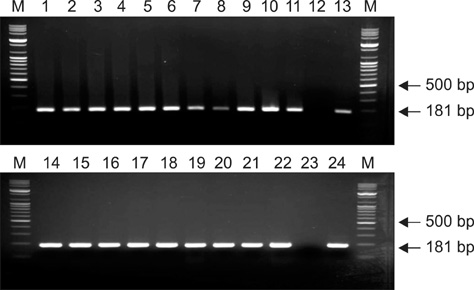
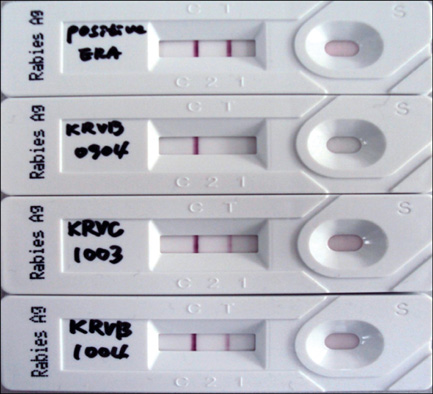
- It is essential to rapidly and precisely diagnose rabies. In this study, we evaluated four diagnostic methods, indirect fluorescent antibody test (FAT), virus isolation (VI), reverse transcriptase polymerase chain reaction (RT-PCR), and rapid immunodiagnostic assay (RIDA), to detect rabies in animal brain homogenates. Out of the 110 animal brain samples tested, 20 (18.2%) were positive for rabies according to the FAT. Compared to the FAT, the sensitivities of VI, RT-PCR, and RIDA were 100, 100, and 95%, respectively. The specificities of VI, RT-PCR and RIDA were found to be 100, 100, and 98.9%, respectively. Rabies viruses circulating in Korea were isolated and propagated in murine neuroblastoma (NG108-15) cells with titers ranging from 101.5 to 104.5 TCID50/mL. Although the RIDA findings did not completely coincide with results obtained from FAT, VI, and RT-PCR, RIDA appears to be a fast and reliable assay that can be used to analyze brain samples. In summary, the results from our study showed that VI, RT-PCR, and RIDA can be used as supplementary diagnostic tools for detecting rabies viruses in both laboratory and field settings.
Keyword
MeSH Terms
-
Animals
Antigens, Viral/blood
Brain/virology
Fluorescent Antibody Technique, Indirect/*veterinary
Immunoassay/*veterinary
RNA, Viral/genetics/isolation & purification
Rabies/diagnosis/*veterinary/virology
Rabies virus/genetics/*isolation & purification
Republic of Korea
Reverse Transcriptase Polymerase Chain Reaction/*veterinary
Sensitivity and Specificity
Figure
Reference
-
1. Biswal M, Ratho R, Mishra B. Usefulness of reverse transcriptase-polymerase chain reaction for detection of rabies RNA in archival samples. Jpn J Infect Dis. 2007. 60:298–299.2. Chhabra M, Mittal V, Jaiswal R, Malik S, Gupta M, Lal S. Development and evaluation of an in vitro isolation of street rabies virus in mouse neuroblastoma cells as compared to conventional tests used for diagnosis of rabies. Indian J Med Microbiol. 2007. 25:263–266.
Article3. David D, Yakobson B, Rotenberg D, Dveres N, Davidson I, Stram Y. Rabies virus detection by RT-PCR in decomposed naturally infected brains. Vet Microbiol. 2002. 87:111–118.
Article4. Hwang EK. Outbreak and control of rabies in animals in Korea. Korean J Vet Public Health. 1995. 19:281–293.5. Hyun BH, Lee KK, Kim IJ, Lee KW, Park HJ, Lee OS, An SH, Lee JB. Molecular epidemiology of rabies virus isolates from South Korea. Virus Res. 2005. 114:113–125.
Article6. Kang B, Oh J, Lee C, Park BK, Park Y, Hong K, Lee K, Cho B, Song D. Evaluation of a rapid immunodiagnostic test kit for rabies virus. J Virol Methods. 2007. 145:30–36.
Article7. Lee KK. Outbreaks and control of animal rabies in Korea. Infect Chemother. 2010. 42:1–5.
Article8. Lembo T, Niezgoda M, Velasco-Villa A, Cleaveland S, Ernest E, Rupprecht CE. Evaluation of a direct, rapid immunohistochemical test for rabies diagnosis. Emerg Infect Dis. 2006. 12:310–313.
Article9. Nishizono A, Khawplod P, Ahmed K, Goto K, Shiota S, Mifune K, Yasui T, Takayama K, Kobayashi Y, Mannen K, Tepsumethanon V, Mitmoonpitak C, Inoue S, Morimoto K. A simple and rapid immunochromatographic test kit for rabies diagnosis. Microbiol Immunol. 2008. 52:243–249.
Article10. Office International des Épizooties (OIE). Manual ofDiagnostic Tests and Vaccines for Terrestrial Animals. 2008. 6th ed. Paris: OIE;304–322.11. Park YJ, Shin MK, Kwon HM. Genetic characterization of rabies virus isolates in Korea. Virus Genes. 2005. 30:341–347.
Article12. Rudd RJ, Trimarchi CV. Comparison of sensitivity of BHK-21 and murine neuroblastoma cells in the isolation of a street strain rabies virus. J Clin Microbiol. 1987. 25:1456–1458.
Article13. Rojas Anaya E, Loza-Rubio E, Banda Ruiz VM, Hernández Baumgarten E. Use of reverse transcription-polymerase chain reaction to determine the stability of rabies virus genome in brains kept at room temperature. J Vet Diagn Invest. 2006. 18:98–101.
Article14. Sacramento D, Bourhy H, Tordo N. PCR technique as an alternative method for diagnosis and molecular epidemiology of rabies virus. Mol Cell Probes. 1991. 5:229–240.
Article15. Tao XY, Niezgoda M, Du JL, Li H, Wang XG, Huang Y, Jiao Y, Cao L, Tang Q, Liang GD. The primary application of direct rapid immunohistochemical test to rabies diagnosis in China. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2008. 22:168–170.16. Tollis M, Buonavoglia C, di Trani L, Vignolo E. Sensitivity of different cell lines for rabies virus isolation. J Vet Med B Infect Dis Vet Public Health. 1988. 35:504–508.
Article17. Trimarchi CV, Nadin-Davis SA. Jackson AC, Wunner WH, editors. Diagnostic evaluation. Rabies. 2007. 2nd ed. London: Academic press;411–462.
Article18. Wacharapluesadee S, Sutipanya J, Damrongwatanapokin S, Phumesin P, Chamnanpood P, Leowijuk C, Hemachudha T. Development of a TaqMan real-time RT-PCR assay for the detection of rabies virus. J Virol Methods. 2008. 151:317–320.
Article19. World Health Organization (WHO). WHO expert committee on rabies. World Health Organ Tech Rep Ser. 1992. 824:1–84.20. Yang J, Hooper DC, Wunner WH, Koprowski H, Dietzschold B, Fu ZF. The specificity of rabies virus RNA encapsidation by nucleoprotein. Virology. 1998. 242:107–117.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The present and future of rabies vaccine in animals
- Conservation of matrix protein genes in rabies viruses circulating in South Korea since 1999
- A Case of Rabies Diagnosis by Skin Biopsy Including Hair Follicles on the Posterior Neck
- Strategies to maintain Korea's animal rabies non-occurrence status
- General Features and Post-Exposure Prophylaxis of Rabies