Yonsei Med J.
2007 Oct;48(5):754-764. 10.3349/ymj.2007.48.5.754.
Bone Marrow Mononuclear Stem Cells Transplanted in Rat Infarct Myocardium Improved the Electrical Conduction without Evidence of Proarrhythmic Effects
- Affiliations
-
- 1Division of Cardiology, Yonsei Cardiovascular Center and Research Institute, Yonsei University College of Medicine, Seoul, Korea. mhlee@yuhs.ac
- 2Department of Cardiovascular Surgery, Yonsei Cardiovascular Center and Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1122611
- DOI: http://doi.org/10.3349/ymj.2007.48.5.754
Abstract
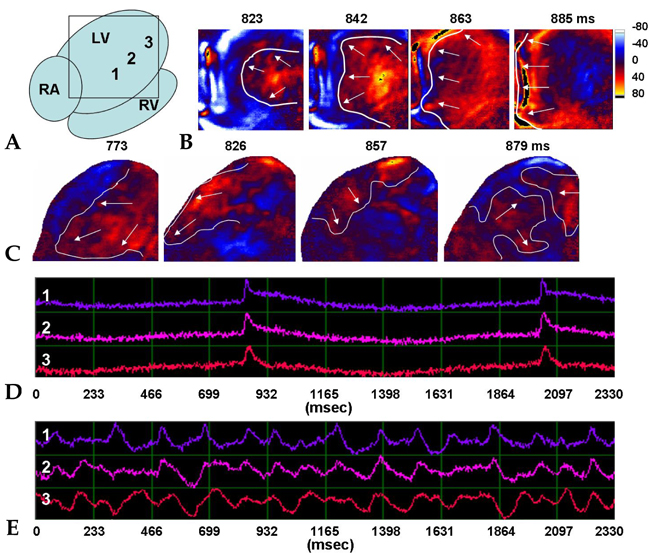
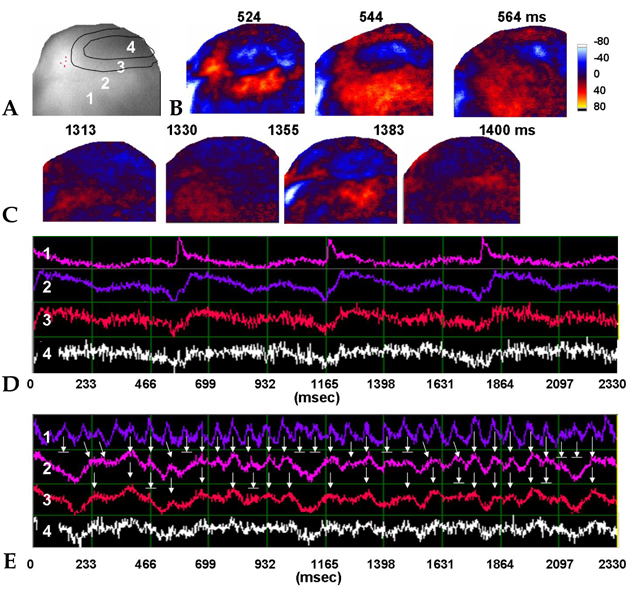
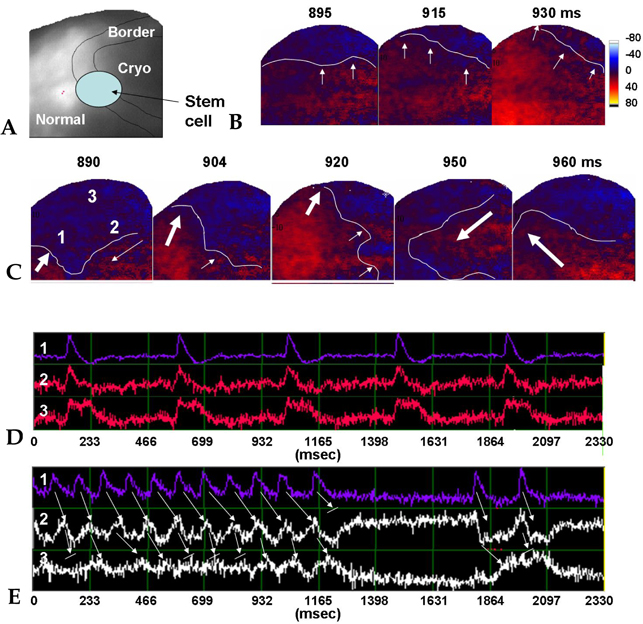
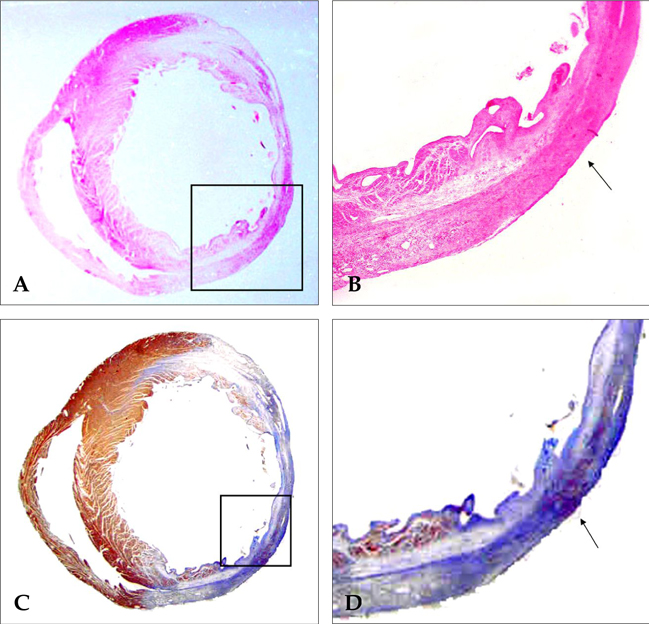
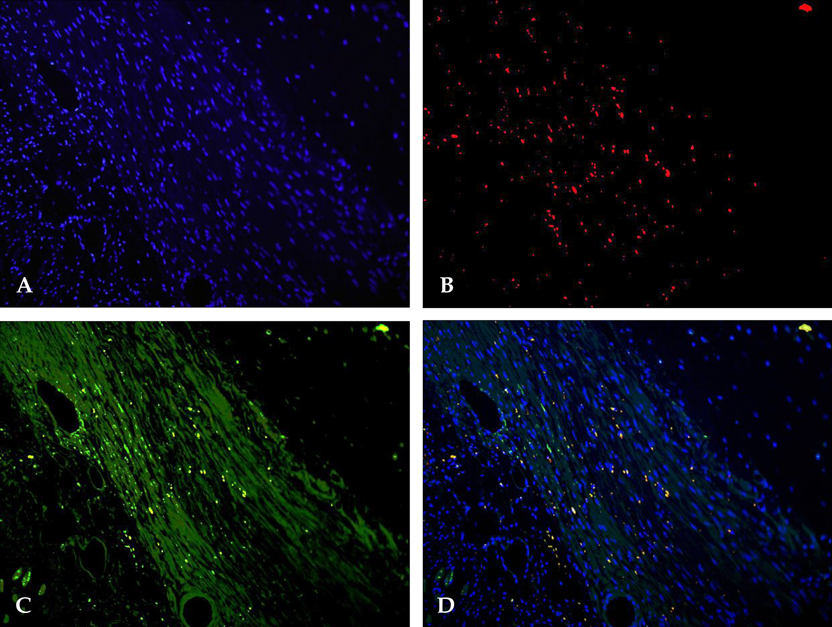
- PURPOSE: The arrhythmogenic effect of stem cells transplantation (SCT) in an infarct myocardium is still unknown. We investigated arrhythmogenicity of SCT in rat cryo-infarct model. MATERIALS AND METHODS: In rat cryo-infarct model, bone marrow mononuclear stem cells (MNSC, 1 x 10(7) cells) were transplanted into the infarct border zone (BZ) of the LV epicardium. We compared the optical mapping and inducibility of ventricular tachycardia/fibrillation (VT/VF) among normal (n=5), cryo-infarct (n=6), and SCT rats (n=6). RESULTS: The VT/VF inducibility was higher in the cryo- infarct (47.2%, p=0.001) and SCT groups (34.6%, p=0.01) than in the normal group (12.8%). The induced VT/VF episodes persisted for more than 2 minutes in 4.3%, 26.4% and 17.3% in the normal, cryo-infarct and SCT group, respectively. In the SCT group, the action potential duration at 70% was shorter at the SCT site than the BZ during SR (75.2 +/- 8.1 vs. 145.6 +/- 4.4 ms, p=0.001) and VT (78.2 +/- 13.0 vs. 125.7 +/- 21.0 ms, p= 0.001). Conduction block was observed at the SCT site and BZ during VT. However, no reentry or ectopic foci were observed around the SCT sites. CONCLUSION: The electrical conduction was improved by SCT without evidence of augmentation of arrhythmia in the rat cryo-infarct model.
Keyword
MeSH Terms
-
Action Potentials
Animals
Arrhythmias, Cardiac/*etiology
Bone Marrow Transplantation/*adverse effects
Disease Models, Animal
Electric Conductivity
Heart Ventricles/pathology/transplantation
Hematopoietic Stem Cell Transplantation/*adverse effects
Myocardial Infarction/pathology/*surgery
Rats
Rats, Sprague-Dawley
Figure
Reference
-
1. Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001. 410:701–705.
Article2. Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001. 104:1046–1052.
Article3. Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004. 428:668–673.
Article4. Yoon YS, Wecker A, Heyd L, Park JS, Tkebuchava T, Kusano K, et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005. 115:326–338.
Article5. Strauer BE, Brehm M, Zeus T, Köstering M, Hernandez A, Sorg RV, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002. 106:1913–1918.
Article6. Assmus B, Schächinger V, Teupe C, Britten M, Lehmann R, Döbert N, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation. 2002. 106:3009–3017.
Article7. Fernández-Avilés F, San Román JA, García-Frade J, Fernández ME, Peñarrubia MJ, de la Fuente L, et al. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res. 2004. 95:742–748.
Article8. Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004. 364:141–148.
Article9. Smits PC, van Geuns RJ, Poldermans D, Bountioukos M, Onderwater EE, Lee CH, et al. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: clinical experience with six-month follow-up. J Am Coll Cardiol. 2003. 42:2063–2069.
Article10. Menasché P, Hagège AA, Scorsin M, Pouzet B, Desnos M, Duboc D, et al. Myoblast transplantation for heart failure. Lancet. 2001. 357:279–280.
Article11. Menasché P, Hagège AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003. 41:1078–1083.
Article12. Ryu JH, Kim IK, Cho SW, Cho MC, Hwang KK, Piao H, et al. Implantation of bone marrow mononuclear cells using injectable fibrin matrix enhances neovascularization in infarcted myocardium. Biomaterials. 2005. 26:319–326.
Article13. Lee MH, Lin SF, Ohara T, Omichi C, Okuyama Y, Chudin E, et al. Effects of diacetyl monoxime and cytochalasin D on ventricular fibrillation in swine right ventricles. Am J Physiol Heart Circ Physiol. 2001. 280:H2689–H2696.
Article14. Garfinkel A, Kim YH, Voroshilovsky O, Qu Z, Kil JR, Lee MH, et al. Preventing ventricular fibrillation by flattening cardiac restitution. Proc Natl Acad Sci U S A. 2000. 97:6061–6066.
Article15. Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the International Society for Heart and Lung Transplantation; endorsed by the Heart Failure Society of America. Circulation. 2001. 104:2996–3007.
Article16. Peters NS. Arrhythmias after cell transplantation for myocardial regeneration: natural history or result of the intervention? J Cardiovasc Electrophysiol. 2005. 16:1255–1257.
Article17. Makkar RR, Lill M, Chen PS. Stem cell therapy for myocardial repair: is it arrhythmogenic? J Am Coll Cardiol. 2003. 42:2070–2072.18. Pinto JM, Boyden PA. Electrical remodeling in ischemia and infarction. Cardiovasc Res. 1999. 42:284–297.
Article19. Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin 43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997. 95:988–996.
Article20. Wit AL, Janse MJ. The ventricular arrhythmias of ischemia and infarction. Electrophysiological mechanisms. 1993. Mount Kisco, NY: Futura Publishing.21. Ursell PC, Gardner PI, Albala A, Fenoglio JJ Jr, Wit AL. Structural and electrophysiological changes in the epicardial border zone of canine myocardial infarcts during infarct healing. Circ Res. 1985. 56:436–451.
Article22. de Bakker JM, van Capelle FJ, Janse MJ, Wilde AA, Coronel R, Becker AE, et al. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988. 77:589–606.
Article23. Callans DJ, Josephson ME. Zipes DP, Jalife J, editors. Ventricular tachycardia associated with coronary artery disease. Cardiac electrophysiology: From cell to Bedside. 1995. 2nd ed. Philadelphia: WB Saunders;732–743.24. Zhang YM, Hartzell C, Narlow M, Dudley SC Jr. Stem cell-derived cardiomyocytes demonstrate arrhythmic potential. Circulation. 2002. 106:1294–1299.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stem Cell Therapy for Patients with Myocardial Infarction
- Clinical Trials of Adult Stem Cell Therapy in Patients with Ischemic Stroke
- Stem Cells for Cardiovascular Disease
- Survival and Graft versus Host Disease in Murine MHC Mismatched Hematopoietic Stem Cell Transplantation with Co-injection of Mesenchymal Stem Cells
- Autologous Bone Marrow Derived Stem Cells for the Treatment of Multiple Sclerosis