J Vet Sci.
2007 Dec;8(4):383-392. 10.4142/jvs.2007.8.4.383.
Enhancement of protective immune responses by oral vaccination with Saccharomyces cerevisiae expressing recombinant Actinobacillus pleuropneumoniae ApxIA or ApxIIA in mice
- Affiliations
-
- 1Department of Infectious Diseases, College of Veterinary Medicine, BK21 for Veterinary Science and KRF Zoonotic Disease Priority Research Institute, Seoul National University, Seoul 151-742, Korea. yoohs@snu.ac.kr
- 2Division of Biological Science, Institute for Molecular Biology and Genetics, Chonbuk National University, Jeonju 561-756, Korea.
- KMID: 1106219
- DOI: http://doi.org/10.4142/jvs.2007.8.4.383
Abstract
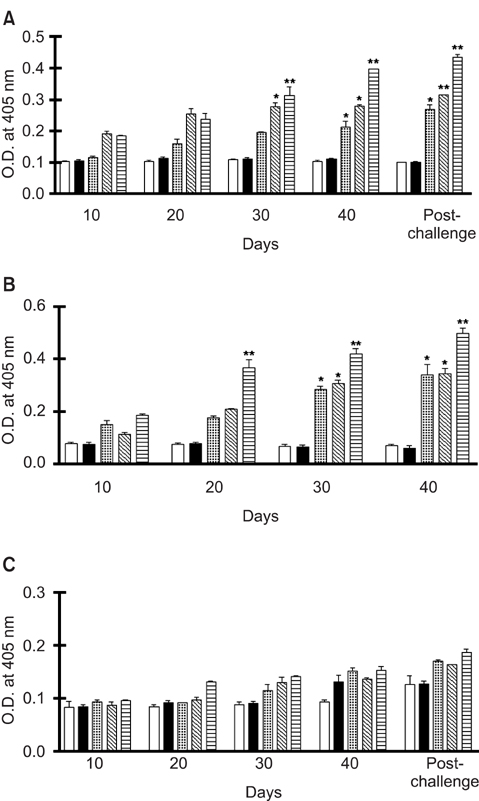
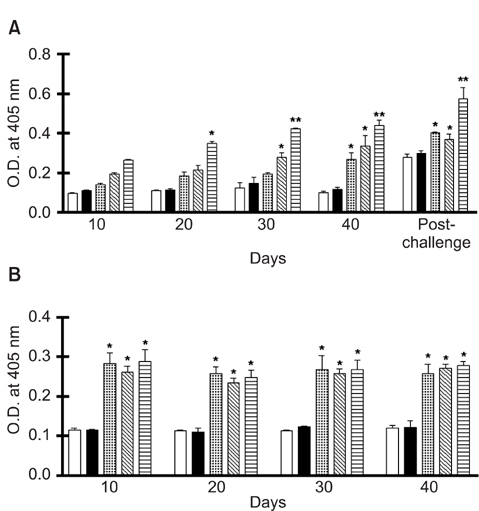
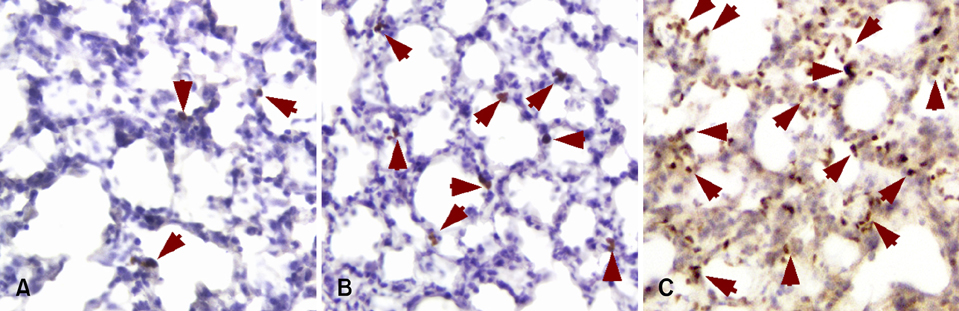
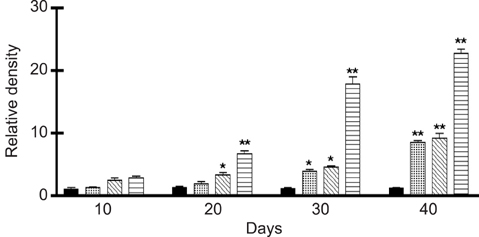
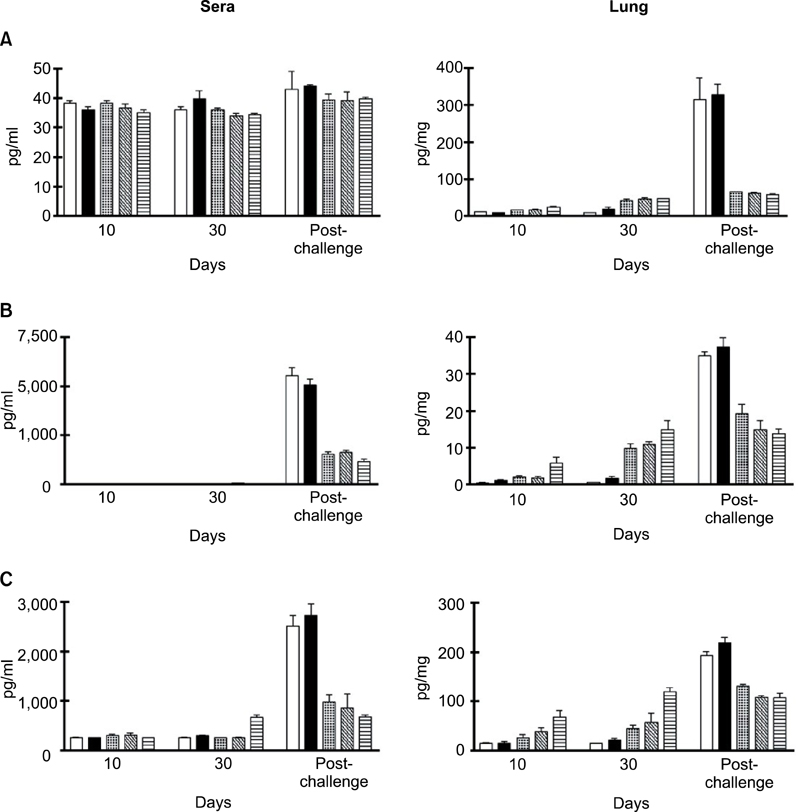
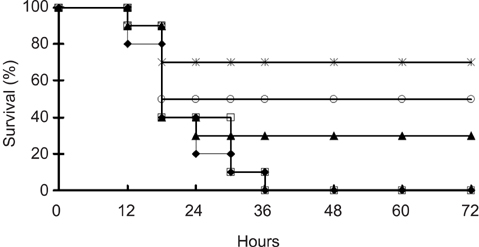
- We previously induced protective immune response by oral immunization with yeast expressing the ApxIIA antigen. The ApxI antigen is also an important factor in the protection against Actinobacillus pleuropneumoniae serotype 5 infection; therefore, the protective immunity in mice following oral immunization with Saccharomyces cerevisiae expressing either ApxIA (group C) or ApxIIA (group D) alone or both (group E) was compared with that in two control groups (group A and B). The immunogenicity of the rApxIA antigen derived from the yeast was confirmed by a high survival rate and an ApxIA-specific IgG antibody response (p < 0.01). The highest systemic (IgG) and local (IgA) humoral immune responses to ApxIA and ApxIIA were detected in group E after the third immunization (p < 0.05). The levels of IL-1beta and IL-6 after challenge with an A. pleuropneumoniae field isolate did not change significantly in the vaccinated groups. The level of TNF-alpha increased in a time-dependent manner in group E but was not significantly different after the challenge. After the challenge, the mice in group E had a significantly lower infectious burden and a higher level of protection than the mice in the other groups (p < 0.05). The survival rate in each group was closely correlated to the immune response and histopathological observations in the lung following the challenge. These results suggested that immunity to the ApxIA antigen is required for optimal protection.
MeSH Terms
-
Actinobacillus Infections/prevention & control
Actinobacillus pleuropneumoniae/genetics/*immunology
Animals
Antibodies, Bacterial/blood
Bacterial Proteins/analysis/*immunology
Cytokines/analysis/blood
Disease Models, Animal
Female
Hemolysin Proteins/analysis/*immunology
Immunoglobulin A/blood/immunology
Intestines/immunology
Lung/cytology/immunology
Mice
Mice, Inbred BALB C
Recombinant Proteins/*immunology
Saccharomyces cerevisiae/*genetics/immunology
Survival Analysis
Time Factors
Vaccination
Vaccines, Synthetic/administration & dosage/*immunology
Figure
Cited by 1 articles
-
Recombinant Kluyveromyces lactis expressing highly pathogenic porcine reproductive and respiratory syndrome virus GP5 elicits mucosal and cell-mediated immune responses in mice
Haiyan Zhao, Yalan Wang, Zhitao Ma, Yongqiang Wang, Wen-hai Feng
J Vet Sci. 2014;15(2):199-208. doi: 10.4142/jvs.2014.15.2.199.
Reference
-
1. Bathurst IC. Protein expression in yeast as an approach to production of recombinant malaria antigens. Am J Trop Med Hyg. 1994. 50:20–26.
Article2. Beier R, Gebert A. Kinetics of particle uptake in the domes of Peyer's patches. Am J Physiol. 1998. 275:G130–G137.
Article3. Blanquet S, Antonelli R, Laforet L, Denis S, Marol-Bonnin S, Alric M. Living recombinant Saccharomyces cerevisiae secreting proteins or peptides as a new drug delivery system in the gut. J Biotechnol. 2004. 110:37–49.
Article4. Blanquet S, Meunier JP, Minekus M, Marol-Bonnin S, Alric M. Recombinant Saccharomyces cerevisiae expressing P450 in artificial digestive systems: a model for biodetoxication in the human digestive environment. Appl Environ Microbiol. 2003. 69:2884–2892.
Article5. Boekema BK, Kamp EM, Smits MA, Smith HE, Stockhofe-Zurwieden N. Both ApxI and ApxII of Actinobacillus pleuropneumoniae serotype 1 are necessary for full virulence. Vet Microbiol. 2004. 100:17–23.
Article6. Bosse JT, Janson H, Sheehan BJ, Beddek AJ, Rycroft AN, Kroll JS, Langford PR. Actinobacillus pleuropneumoniae: pathobiology and pathogenesis of infection. Microbes Infect. 2002. 4:225–235.7. Bouvet JP, Decroix N, Pamonsinlapatham P. Stimulation of local antibody production: parenteral or mucosal vaccination? Trends Immunol. 2002. 23:209–213.
Article8. Bowersock TL, Shalaby WS, Levy M, Samuels ML, Lallone R, White MR, Borie DL, Lehmeyer J, Park K. Evaluation of an orally administered vaccine, using hydrogels containing bacterial exotoxins of Pasteurella haemolytica, in cattle. Am J Vet Res. 1994. 55:502–509.9. Broug-Holub E, Toews GB, van Iwaarden JF, Strieter RM, Kunkel SL, Paine R, Standiford TJ. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun. 1997. 65:1139–1146.
Article10. Choi C, Kwon D, Min K, Chae C. In-situ hybridization for the detection of inflammatory cytokines (IL-1, TNF-alpha and IL-6) in pigs naturally infected with Actinobacillus pleuropneumoniae. J Comp Pathol. 1999. 121:349–356.
Article11. Cox E, Van der Stedea Y, Verdonck F, Snoeck V, Van den Broeck W, Goddeeris B. Oral immunisation of pigs with fimbrial antigens of enterotoxigenic E. coli: an interesting model to study mucosal immune mechanisms. Vet Immunol Immunopathol. 2002. 87:287–290.
Article12. Dietrich G, Griot-Wenk M, Metcalfe IC, Lang AB, Viret JF. Experience with registered mucosal vaccines. Vaccine. 2003. 21:678–683.
Article13. Externest D, Meckelein B, Schmidt MA, Frey A. Correlations between antibody immune responses at different mucosal effector sites are controlled by antigen type and dosage. Infect Immun. 2000. 68:3830–3839.
Article14. Fernandez MI, Pedron T, Tournebize R, Olivo-Marin JC, Sansonetti PJ, Phalipon A. Anti-inflammatory role for intracellular dimeric immunoglobulin a by neutralization of lipopolysaccharide in epithelial cells. Immunity. 2003. 18:739–749.
Article15. Frey J. Virulence in Actinobacillus pleuropneumoniae and RTX toxins. Trends Microbiol. 1995. 3:257–261.16. Fuller TE, Martin S, Teel JF, Alaniz GR, Kennedy MJ, Lowery DE. Identification of Actinobacillus pleuropneumoniae virulence genes using signature-tagged mutagenesis in a swine infection model. Microb Pathog. 2000. 29:39–51.
Article17. Haga Y, Ogino S, Ohashi S, Ajito T, Hashimoto K, Sawada T. Protective efficacy of an affinity-purified hemolysin vaccine against experimental swine pleuropneumonia. J Vet Med Sci. 1997. 59:115–120.
Article18. Hensel A, van Leengoed LA, Szostak M, Windt H, Weissenbock H, Stockhofe-Zurwieden N, Katinger A, Stadler M, Ganter M, Bunka S, Pabst R, Lubitz W. Induction of protective immunity by aerosol or oral application of candidate vaccines in a dose-controlled pig aerosol infection model. J Biotechnol. 1996. 44:171–181.
Article19. Lauterslager TG, Hilgers LA. Efficacy of oral administration and oral intake of edible vaccines. Immunol Lett. 2002. 84:185–190.
Article20. Lauterslager TG, Stok W, Hilgers LA. Improvement of the systemic prime/oral boost strategy for systemic and local responses. Vaccine. 2003. 21:1391–1399.
Article21. Losinger WC. Economic impacts of reduced pork production associated with the diagnosis of Actinobacillus pleuropneumoniae on grower/finisher swine operations in the United States. Prev Vet Med. 2005. 68:181–193.
Article22. Ogra PL, Faden H, Welliver RC. Vaccination strategies for mucosal immune responses. Clin Microbiol Rev. 2001. 14:430–445.
Article23. Olas K, Butterweck H, Teschner W, Schwarz HP, Reipert B. Immunomodulatory properties of human serum immunoglobulin A: anti-inflammatory and pro-inflammatory activities in human monocytes and peripheral blood mononuclear cells. Clin Exp Immunol. 2005. 140:478–490.
Article24. Pabst R, Binns RM. The immune system of the respiratory tract in pigs. Vet Immunol Immunopathol. 1994. 43:151–156.
Article25. Pascual DW, Trunkle T, Sura J. Fimbriated Salmonella enterica serovar typhimurium abates initial inflammatory responses by macrophages. Infect Immun. 2002. 70:4273–4281.
Article26. Prideaux CT, Lenghaus C, Krywult J, Hodgson AL. Vaccination and protection of pigs against pleuropneumonia with a vaccine strain of Actinobacillus pleuropneumoniae produced by site-specific mutagenesis of the ApxII operon. Infect Immun. 1999. 67:1962–1966.
Article27. Rappuoli R, Pizza M, Douce G, Dougan G. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol Today. 1999. 20:493–500.
Article28. Reimer D, Frey J, Jansen R, Veit HP, Inzana TJ. Molecular investigation of the role of ApxI and ApxII in the virulence of Actinobacillus pleuropneumoniae serotype 5. Microb Pathog. 1995. 18:197–209.
Article29. Saegusa S, Totsuka M, Kaminogawa S, Hosoi T. Candida albicans and Saccharomyces cerevisiae induce interleukin-8 production from intestinal epithelial-like Caco-2 cells in the presence of butyric acid. FEMS Immunol Med Microbiol. 2004. 41:227–235.
Article30. Schreuder MP, Deen C, Boersma WJ, Pouwels PH, Klis FM. Yeast expressing hepatitis B virus surface antigen determinants on its surface: implications for a possible oral vaccine. Vaccine. 1996. 14:383–388.
Article31. Schreuder MP, Mooren AT, Toschka HY, Verrips CT, Klis FM. Immobilizing proteins on the surface of yeast cells. Trends Biotechnol. 1996. 14:115–120.
Article32. Seah JN, Frey J, Kwang J. The N-terminal domain of RTX toxin ApxI of Actinobacillus pleuropneumoniae elicits protective immunity in mice. Infect Immun. 2002. 70:6464–6467.
Article33. Shin SJ, Cho YW, Yoo HS. Cloning, sequencing and expression of apxIA, IIA, IIIA of Actinobacillus pleuropneumoniae isolated in Korea. Korean J Vet Res. 2003. 43:247–253.34. Shin SJ, Bae JL, Cho YW, Lee DY, Kim DH, Yang MS, Jang YS, Yoo HS. Induction of antigen-specific immune responses by oral vaccination with Saccharomyces cerevisiae expressing Actinobacillus pleuropneumoniae ApxIIA. FEMS Immunol Med Microbiol. 2005. 43:155–164.
Article35. Shin SJ, Bae JL, Cho YW, Yang MS, Kim DH, Jang YS, Yoo HS. Expression of apxIA of Actinobacillus pleuropneumoniae in Saccharomyces cerevisiae. J Vet Sci. 2003. 4:225–228.
Article36. Shin SJ, Wu CW, Steinberg H, Talaat AM. Identification of novel virulence determinants in Mycobacterium paratuberculosis by screening a library of insertional mutants. Infect Immun. 2006. 74:3825–3833.
Article37. Silin DS, Lyubomska V. Overcoming immune tolerance during oral vaccination against Actinobacillus pleuropneumoniae. J Vet Med B Infect Dis Vet Public Health. 2002. 49:169–175.
Article38. Stubbs AC, Martin KS, Coeshott C, Skaates SV, Kuritzkes DR, Bellgrau D, Franzusoff A, Duke RC, Wilson CC. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat Med. 2001. 7:625–629.
Article39. Tonpitak W, Baltes N, Hennig-Pauka I, Gerlach GF. Construction of an Actinobacillus pleuropneumoniae serotype 2 prototype live negative-marker vaccine. Infect Immun. 2002. 70:7120–7125.
Article40. Williams AE, Edwards L, Humphreys IR, Snelgrove R, Rae A, Rappuoli R, Hussell T. Innate imprinting by the modified heat-labile toxin of Escherichia coli (LTK63) provides generic protection against lung infectious disease. J Immunol. 2004. 173:7435–7443.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A predictive model for the level of sIgA based on IgG levels following the oral administration of antigens expressed in Sacchromyces cerevisiae
- Expression of apxIA of Actinobacillus pleuropneumoniae in Saccharomyces cerevisiae
- Generation of transgenic corn-derived Actinobacillus pleuropneumoniae ApxIIA fused with the cholera toxin B subunit as a vaccine candidate
- Development of Actinobacillus pleuropneumoniae ApxI, ApxII, and ApxIII-specific ELISA methods for evaluation of vaccine efficiency
- Prevalence and Characterization of Actinobacillus pleuropneumoniae Isolated from Korean Pigs