J Vet Sci.
2007 Sep;8(3):213-218. 10.4142/jvs.2007.8.3.213.
Effects of endocrine disrupting chemicals on expression of phospholipid hydroperoxide glutathione peroxidase mRNA in rat testes
- Affiliations
-
- 1College of Veterinary Medicine and Research Institute of Veterinary Medicine, Chungbuk National University, Cheongju 361-763, Korea. synam@cbu.ac.kr
- 2College of Pharmacy, Chungbuk National University, Cheongju 361-763, Korea.
- 3Department of Biological Science, College of Natural Sciences, Wonkwang University, Iksan 570-749, Korea.
- KMID: 1090793
- DOI: http://doi.org/10.4142/jvs.2007.8.3.213
Abstract
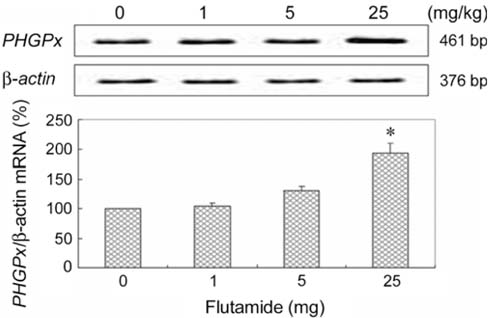
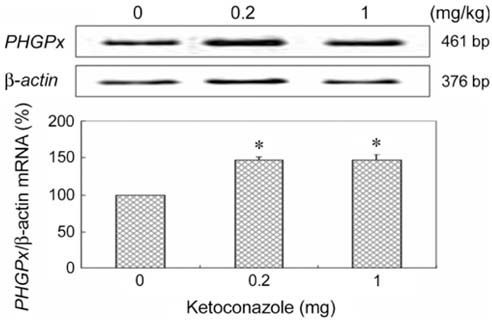
- Phospholipid hydroperoxide glutathione peroxidase(PHGPx), an antioxidative selenoprotein, is modulated byestrogen in the testis and oviduct. To examine whetherpotential endocrine disrupting chemicals (EDCs) affectthe microenvironment of the testes, the expression patternsof PHGPx mRNA and histological changes were analyzedin 5-week-old Sprague-Dawley male rats exposed to severalEDCs such as an androgenic compound [testosterone (50,200, and 1,000microg/kg)], anti-androgenic compounds [flutamide(1, 5, and 25mg/kg), ketoconazole (0.2 and 1mg/kg), anddiethylhexyl phthalate (10, 50, and 250mg/kg)], andestrogenic compounds [nonylphenol (10, 50, 100, and 250mg/kg), octylphenol (10, 50, and 250mg/kg), and diethyl-stilbestrol (10, 20, and 40microg/kg)] daily for 3 weeks via oraladministration. Mild proliferation of germ cells andhyperplasia of interstitial cells were observed in the testesof the flutamide-treated group and deletion of thegerminal epithelium and sloughing of germ cells wereobserved in testes of the diethylstilbestrol-treated group.Treatment with testosterone was shown to slightly decreasePHGPx mRNA levels in testes by the reverse transcription-polymerase chain reaction. However, anti-androgeniccompounds (flutamide, ketoconazole, and diethylhexylphthalate) and estrogenic compounds (nonylphenol,octylphenol, and diethylstilbestrol) significantly up-regulated PHGPx mRNA in the testes (p<0.05). Thesefindings indicate that the EDCs might have a detrimentaleffect on spermatogenesis via abnormal enhancement ofPHGPx expression in testes and that PHGPx is useful as abiomarker for toxicity screening of estrogenic or anti-androgenic EDCs in testes.
Keyword
MeSH Terms
-
Androgen Antagonists/pharmacology
Animals
Diethylhexyl Phthalate/pharmacology
Diethylstilbestrol/pharmacology
Endocrine Disruptors/*pharmacology
Estrogens, Non-Steroidal/pharmacology
Flutamide/pharmacology
Glutathione Peroxidase/*biosynthesis/genetics
Histocytochemistry
Ketoconazole/pharmacology
Male
Phenols/pharmacology
RNA, Messenger/*biosynthesis/genetics
Rats
Rats, Sprague-Dawley
Reverse Transcriptase Polymerase Chain Reaction
Spermatogenesis/drug effects
Testis/*drug effects/*enzymology
Testosterone/pharmacology
Figure
Reference
-
1. Albertson BD, Frederick KL, Maronian NC, Feuillan P, Schorer S, Dunn JF, Loriaux DL. The effect of ketoconazole on steroidogenesis: I. Leydig cell enzyme activity in vitro. Res Commun Chem Pathol Pharmacol. 1988. 61:17–26.2. Barlow NJ, Phillips SL, Wallace DG, Sar M, Gaido KW, Foster PM. Quantitative changes in gene expression in fetal rat testes following exposure to di(n-butyl) phthalate. Toxicol Sci. 2003. 73:431–441.
Article3. Baskin LS, Himes K, Colborn T. Hypospadias and endocrine disruption: is there a connection? Environ Health Perspect. 2001. 109:1175–1183.
Article4. Boockfor FR, Blake CA. Chronic administration of 4-tert-octylphenol to adult male rats causes shrinkage of the testes and male accessory sex organs, disrupts spermatogenesis, and increases the incidence of sperm deformities. Biol Reprod. 1997. 57:267–277.
Article5. Brigelius-Flohé R. Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med. 1999. 27:951–965.
Article6. Brigelius-Flohé R, Aumann KD, Blöcker H, Gross G, Kiess M, Klöppel KD, Maiorino M, Roveri A, Schuckelt R, Ursini F, Wingender E, Flohé L. Phospholipid-hydroperoxide glutathione peroxidase. Genomic DNA, cDNA, and deduced amino acid sequence. J Biol Chem. 1994. 269:7342–7348.
Article7. Couse JF, Dixon D, Yates M, Moore AB, Ma L, Maas R, Korach KS. Estrogen receptor-alpha knockout mice exhibit resistance to the developmental effects of neonatal diethylstilbestrol exposure on the female reproductive tract. Dev Biol. 2001. 238:224–238.
Article8. Couse JF, Korach KS. Estrogen receptor-α mediates the detrimental effects of neonatal diethylstilbestrol (DES) exposure in the murine reproductive tract. Toxicology. 2004. 205:55–63.
Article9. Fisher JS. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004. 127:305–315.
Article10. Giger W, Brunner PH, Schaffner C. 4-Nonylphenol in sewage sludge: accumulation of toxic metabolites from nonionic surfactants. Science. 1984. 225:623–625.
Article11. Gray LE, Ostby JS, Kelce WR. Developmental effects of an environmental antiandrogen: the fungicide vinclozolin alters sex differentiation of the male rat. Toxicol Appl Pharmacol. 1994. 129:46–52.
Article12. Hotchkiss AK, Ostby JS, Vandenbergh JG, Gray LE. An environmental antiandrogen, vinclozolin, alters the organization of play behavior. Physiol Behav. 2003. 79:151–156.
Article13. Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med. 2003. 34:145–169.
Article14. Kim HH, Kwak DH, Yon JM, Baek IJ, Lee SR, Lee JE, Nahm SS, Jeong JH, Lee BJ, Yun YW, Nam SY. Differential expression of 3 β-hydroxysteroid dehydrogenase mRNA in rat testes exposed to endocrine disruptors. J Reprod Dev. 2007. 53:465–471.
Article15. Knopp EA, Arndt TL, Eng KL, Caldwell M, LeBoeuf RC, Deeb SS, O'Brien KD. Murine phospholipid hydroperoxide glutathione peroxidase: cDNA sequence, tissue expression, and mapping. Mamm Genome. 1999. 10:601–605.
Article16. Korach KS, Chae K, Gibson M, Curtis S. Estrogen receptor stereochemistry: ligand binding and hormonal responsiveness. Steroids. 1991. 56:263–270.
Article17. Lapointe J, Kimmins S, Maclaren LA, Bilodeau JF. Estrogen selectively up-regulates the phospholipid hydroperoxide glutathione peroxidase in the oviducts. Endocrinology. 2005. 146:2583–2592.
Article18. LeBlanc GA, Bain LJ, Wilson VS. Pesticides: multiple mechanisms of demasculinization. Mol Cell Endocrinol. 1997. 126:1–5.
Article19. Maiorino M, Wissing JB, Brigelius-Flohé R, Calabrese F, Roveri A, Steinert P, Ursini F, Flohé L. Testosterone mediates expression of the selenoprotein PHGPx by induction of spermatogenesis and not by direct transcriptional gene activation. FASEB J. 1998. 12:1359–1370.
Article20. Majdic G, Sharpe RM, Saunders PT. Maternal oestrogen/xenoestrogen exposure alters expression of steroidogenic factor-1 (SF-1/Ad4BP) in the fetal rat testis. Mol Cell Endocrinol. 1997. 127:91–98.
Article21. Miossec P, Archambeaud-Mouveroux F, Teissier MP. Inhibition of steroidogenesis by ketoconazole. Therapeutic uses. Ann Endocrinol (Paris). 1997. 58:494–502.22. Mylchreest E, Sar M, Cattley RC, Foster PM. Disruption of androgen-regulated male reproductive development by di(n-butyl) phthalate during late gestation in rats is different from flutamide. Toxicol Appl Pharmacol. 1999. 156:81–95.
Article23. Nam SY, Baek IJ, Lee BJ, In CH, Jung EY, Yon JM, Ahn B, Kang JK, Yu WJ, Yun YW. Effects of 17β-estradiol and tamoxifen on the selenoprotein phospholipid hydroperoxide glutathione peroxidase (PHGPx) mRNA expression in male reproductive organs of rats. J Reprod Dev. 2003. 49:389–396.
Article24. Nam SY, Fujisawa M, Kim JS, Kurohmaru M, Hayashi Y. Expression pattern of phospholipid hydroperoxide glutathione peroxidase messenger ribonucleic acid in mouse testis. Biol Reprod. 1998. 58:1272–1276.
Article25. Ohsako S, Kubota K, Kurosawa S, Takeda K, Qing W, Ishimura R, Tohyama C. Alterations of gene expression in adult male rat testis and pituitary shortly after subacute administration of the antiandrogen flutamide. J Reprod Dev. 2003. 49:275–290.
Article26. Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, Gray LE. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci. 2000. 58:339–349.
Article27. Pelletier G, Labrie C, Labrie F. Localization of oestrogen receptor α, oestrogen receptor β and androgen receptors in the rat reproductive organs. J Endocrinol. 2000. 165:359–370.
Article28. Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with αERKO and βERKO mice. Cancer Res. 2001. 61:6089–6097.29. Roveri A, Casasco A, Maiorino M, Dalan P, Calligaro A, Ursini F. Phospholipid hydroperoxide glutathione peroxidase of rat testis. Gonadotropin dependence and immunocytochemical identification. J Biol Chem. 1992. 267:6142–6146.
Article30. Sharpe RM, Irvine DS. How strong is the evidence of a link between environmental chemicals and adverse effects on human reproductive health? BMJ. 2004. 328:447–451.
Article31. Thomas JP, Maiorino M, Ursini F, Girotti AW. Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation. In situ reduction of phospholipid and cholesterol hydroperoxides. J Biol Chem. 1990. 265:454–461.
Article32. Thompson CJ, Ross SM, Gaido KW. Di(n-butyl) phthalate impairs cholesterol transport and steroidogenesis in the fetal rat testis through a rapid and reversible mechanism. Endocrinology. 2004. 145:1227–1237.
Article33. Viguier-Martinez MC, Hochereau-de Reviers MT, Perreau C. Effects of flutamide or of supplementation with testosterone in prepubertal male rats prenatally treated with busulfan. Acta Endocrinol (Copenh). 1985. 109:550–557.
Article34. White R, Jobling S, Hoare SA, Sumpter JP, Parker MG. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994. 135:175–182.
Article35. Yagi K, Komura S, Kojima H, Sun Q, Nagata N, Ohishi N, Nishikimi M. Expression of human phospholipid hydroperoxide glutathione peroxidase gene for protection of host cells from lipid hydroperoxide-mediated injury. Biochem Biophys Res Commun. 1996. 219:486–491.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ffects of Endocrine-Disrupting Chemicals on Human Health
- Epigenetic control of endocrine disrupting chemicals on gynecological disease: Focused on phthalates
- Childhood obesity and endocrine disrupting chemicals
- Association between Exposure to Endocrine Disrupting Chemicals in Breast Milk and Maternal Lifestyle Factor
- Endocrine disrupting chemicals and environmental diseases