Ann Pediatr Endocrinol Metab.
2017 Dec;22(4):219-225. 10.6065/apem.2017.22.4.219.
Childhood obesity and endocrine disrupting chemicals
- Affiliations
-
- 1Department of Internal Medicine, Eulji General Hospital, Eulji University School of Medicine, Seoul, Korea. hkleemd@eulji.ac.kr
- KMID: 2400777
- DOI: http://doi.org/10.6065/apem.2017.22.4.219
Abstract
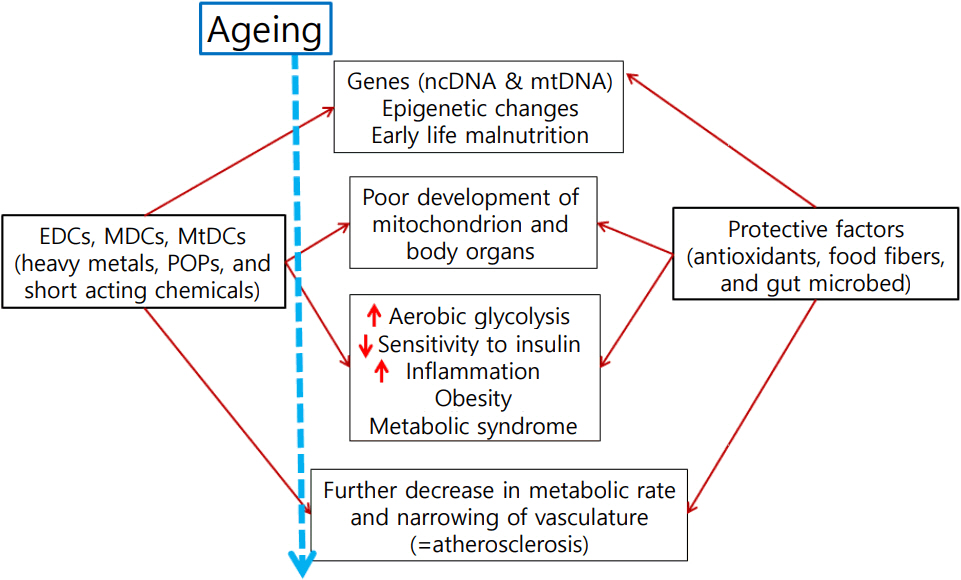
- The prevalence of obesity around the world has increased sharply. Strong evidence has emerged over the last decades that human exposure to numerous endocrine disrupting chemicals (EDCs) is the cause of obesity and obesity-related metabolic diseases. Many EDCs are manmade chemicals that are released into the environment. EDCs are exogenous compounds that interfere with hormonal regulation and normal endocrine systems, thereby affecting the health of animals and humans. The number of chemicals belonging to EDCs is increasing and some of them are very stable; they persist in the environment (persistent organic pollutants). Although they are banned, their concentrations have been continuously increasing over time. This review gives a brief introduction to common EDCs, and evidence of harmful effects of EDCs on obesity-related diseases; we focus in particular on EDCs' role in causing mitochondrial dysfunction.
MeSH Terms
Figure
Reference
-
References
1. James WP. WHO recognition of the global obesity epidemic. Int J Obes (Lond). 2008; 32 Suppl 7:S120–6.
Article2. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011- 2012. JAMA. 2014; 311:806–14.
Article3. Ha KH, Kim DJ. Epidemiology of childhood obesity in Korea. Endocrinol Metab (Seoul). 2016; 31:510–8.
Article4. Min J, Chiu DT, Wang Y. Variation in the heritability of body mass index based on diverse twin studies: a systematic review. Obes Rev. 2013; 14:871–82.
Article5. Sorensen TI, Price RA, Stunkard AJ, Schulsinger F. Genetics of obesity in adult adoptees and their biological siblings. BMJ. 1989; 298:87–90.
Article6. Baillie-Hamilton PF. Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med. 2002; 8:185–92.
Article7. Neel BA, Sargis RM. The paradox of progress: environmental disruption of metabolism and the diabetes epidemic. Diabetes. 2011; 60:1838–48.
Article8. Brown RE, Sharma AM, Ardern CI, Mirdamadi P, Mirdamadi P, Kuk JL. Secular differences in the association between caloric intake, macronutrient intake, and physical activity with obesity. Obes Res Clin Pract. 2016; 10:243–55.9. Lind L, Lind PM, Lejonklou MH, Dunder L, Bergman A, Guerrero-Bosagna C, et al. Uppsala consensus statement on environmental contaminants and the global obesity epidemic. Environ Health Perspect. 2016; 124:A81–3.
Article10. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009; 30:293–342.
Article11. Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015; 36:E1–150.
Article12. Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017; 68:3–33.
Article13. Germain P, Staels B, Dacquet C, Spedding M, Laudet V. Overview of nomenclature of nuclear receptors. Pharmacol Rev. 2006; 58:685–704.
Article14. Tabb MM, Blumberg B. New modes of action for endocrine-disrupting chemicals. Mol Endocrinol. 2006; 20:475–82.
Article15. Park WH, Jun DW, Kim JT, Jeong JH, Park H, Chang YS, et al. Novel cell-based assay reveals associations of circulating serum AhR-ligands with metabolic syndrome and mitochondrial dysfunction. Biofactors. 2013; 39:494–504.
Article16. Hwang HJ, Dornbos P, Steidemann M, Dunivin TK, Rizzo M, LaPres JJ. Mitochondrial-targeted aryl hydrocarbon receptor and the impact of 2,3,7,8-tetrachlorodibenzop- dioxin on cellular respiration and the mitochondrial proteome. Toxicol Appl Pharmacol. 2016; 304:121–32.17. Golub M, Doherty J. Triphenyltin as a potential human endocrine disruptor. J Toxicol Environ Health B Crit Rev. 2004; 7:281–95.
Article18. Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid X receptor pathway. Mol Pharmacol. 2005; 67:766–74.
Article19. Grun F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, et al. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol. 2006; 20:2141–55.
Article20. Maechler P, Wollheim CB. Mitochondrial function in normal and diabetic beta-cells. Nature. 2001; 414:807–12.
Article21. Silva JP, Kohler M, Graff C, Oldfors A, Magnuson MA, Berggren PO, et al. Impaired insulin secretion and beta-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nat Genet. 2000; 26:336–40.
Article22. Soejima A, Inoue K, Takai D, Kaneko M, Ishihara H, Oka Y, et al. Mitochondrial DNA is required for regulation of glucose-stimulated insulin secretion in a mouse pancreatic beta cell line, MIN6. J Biol Chem. 1996; 271:26194–9.
Article23. Lee HK, Song JH, Shin CS, Park DJ, Park KS, Lee KU, et al. Decreased mitochondrial DNA content in peripheral blood precedes the development of non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1998; 42:161–7.
Article24. Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003; 300:1140–2.
Article25. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004; 350:664–71.
Article26. Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005; 115:3587–93.
Article27. Park KS, Chan JC, Chuang LM, Suzuki S, Araki E, Nanjo K, et al. A mitochondrial DNA variant at position 16189 is associated with type 2 diabetes mellitus in Asians. Diabetologia. 2008; 51:602–8.
Article28. Fuku N, Park KS, Yamada Y, Nishigaki Y, Cho YM, Matsuo H, et al. Mitochondrial haplogroup N9a confers resistance against type 2 diabetes in Asians. Am J Hum Genet. 2007; 80:407–15.
Article29. Solomon KR, Giesy JP, LaPoint TW, Giddings JM, Richards RP. Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem. 2013; 32:10–1.
Article30. Lim S, Ahn SY, Song IC, Chung MH, Jang HC, Park KS, et al. Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. PLoS One. 2009; 4:e5186.
Article31. Kleiber M. Body size and metabolic rate. Physiol Rev. 1947; 27:511–41.
Article32. West GB, Brown JH. The origin of allometric scaling laws in biology from genomes to ecosystems: towards a quantitative unifying theory of biological structure and organization. J Exp Biol. 2005; 208:1575–92.
Article33. Dulloo AG, Jacquet J, Montani JP, Schutz Y. Adaptive thermogenesis in human body weight regulation: more of a concept than a measurable entity? Obes Rev. 2012; 13 Suppl 2:105–21.34. Angle BM, Do RP, Ponzi D, Stahlhut RW, Drury BE, Nagel SC, et al. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod Toxicol. 2013; 42:256–68.
Article35. Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013; 8:e55387.
Article36. Skinner MK, Manikkam M, Tracey R, Guerrero- Bosagna C, Haque M, Nilsson EE. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med. 2013; 11:228.
Article37. Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S, et al. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ Health Perspect. 2009; 117:1549–55.
Article38. van Esterik JC, Verharen HW, Hodemaekers HM, Gremmer ER, Nagarajah B, Kamstra JH, et al. Compound-and sex-specific effects on programming of energy and immune homeostasis in adult C57BL/6JxFVB mice after perinatal TCDD and PCB 153. Toxicol Appl Pharmacol. 2015; 289:262–75.39. Hao C, Cheng X, Guo J, Xia H, Ma X. Perinatal exposure to diethyl-hexyl-phthalate induces obesity in mice. Front Biosci (Elite Ed). 2013; 5:725–33.
Article40. Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol. 2009; 304:97–105.
Article41. La Merrill M, Karey E, Moshier E, Lindtner C, La Frano MR, Newman JW, et al. Perinatal exposure of mice to the pesticide DDT impairs energy expenditure and metabolism in adult female offspring. PLoS One. 2014; 9:e103337.
Article42. Mackay H, Patterson ZR, Khazall R, Patel S, Tsirlin D, Abizaid A. Organizational effects of perinatal exposure to bisphenol-A and diethylstilbestrol on arcuate nucleus circuitry controlling food intake and energy expenditure in male and female CD-1 mice. Endocrinology. 2013; 154:1465–75.
Article43. Landrigan PJ, Goldman LR. Children's vulnerability to toxic chemicals: a challenge and opportunity to strengthen health and environmental policy. Health Aff (Millwood). 2011; 30:842–50.
Article44. Xu W, Wang X, Cai Z. Analytical chemistry of the persistent organic pollutants identified in the Stockholm Convention: a review. Anal Chim Acta. 2013; 790:1–13.
Article45. Murk AJ, Legler J, Denison MS, Giesy JP, van de Guchte C, Brouwer A. Chemical-activated luciferase gene expression (CALUX): a novel in vitro bioassay for Ah receptor active compounds in sediments and pore water. Fundam Appl Toxicol. 1996; 33:149–60.46. Nebert DW. Aryl hydrocarbon receptor (AHR): "pioneer member" of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of "sensors" of foreign and endogenous signals. Prog Lipid Res. 2017; 67:38–57.
Article47. Roh E, Kwak SH, Jung HS, Cho YM, Pak YK, Park KS, et al. Serum aryl hydrocarbon receptor ligand activity is associated with insulin resistance and resulting type 2 diabetes. Acta Diabetol. 2015; 52:489–95.
Article48. Trasande L, Lampa E, Lind L, Lind PM. Population attributable risks and costs of diabetogenic chemical exposures in the elderly. J Epidemiol Community Health. 2017; 71:111–4.
Article49. Lee HK, Shim EB. Extension of the mitochondria dysfunction hypothesis of metabolic syndrome to atherosclerosis with emphasis on the endocrine-disrupting chemicals and biophysical laws. J Diabetes Investig. 2013; 4:19–33.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ffects of Endocrine-Disrupting Chemicals on Human Health
- Early-life exposure to endocrine disrupting chemicals associates with childhood obesity
- Endocrine disrupting chemicals and environmental diseases
- Epigenetic control of endocrine disrupting chemicals on gynecological disease: Focused on phthalates
- Association between Exposure to Endocrine Disrupting Chemicals in Breast Milk and Maternal Lifestyle Factor