J Vet Sci.
2007 Jun;8(2):131-137. 10.4142/jvs.2007.8.2.131.
Biological characteristics of Chinese hamster ovary cells transfected with bovine Prnp
- Affiliations
-
- 1Department of Infectious Diseases, KRF Zoonotic Disease Priority Research Institute and BK21 Program for Veterinary Science, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. yoohs@snu.ac.kr
- KMID: 1089663
- DOI: http://doi.org/10.4142/jvs.2007.8.2.131
Abstract
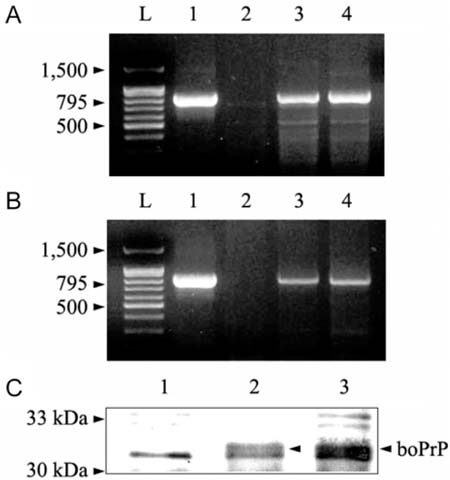
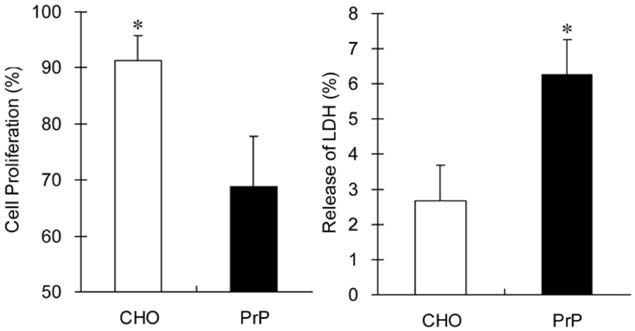
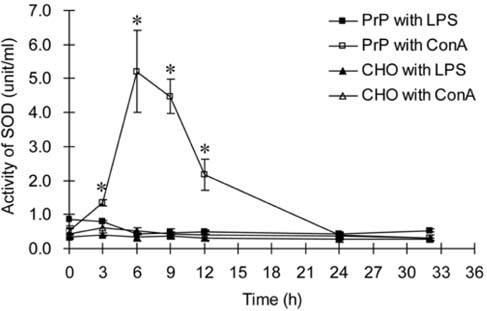
- A normal prion protein (PrPc) is converted to a proteaseresistant isoform by an apparent self-propagating activity in transmissible spongiform encephalopathy, a neurodegenerative disease. The cDNA encoding open reading frame (ORF) of the bovine prion protein gene (Prnp) was cloned from Korean cattle by PCR, and was transfected into Chinese hamster ovary (CHO-K1) cells using lipofectamine. The gene expression of the cloned cDNA was confirmed by RT-PCR and Western blotting with the monoclonal antibody, 6H4. Cellular changes in the transfected CHO-K1 cells were investigated using parameters such as MTT, lactate dehydrogenase (LDH), and superoxide dismutase (SOD) activities, as well as nitric oxide (NO) production, and an apoptosis assay. In the MTT and LDH assays, the bovine PrnP-transfectant showed a lower proliferation rate than the wild-type (p < 0.05). Production of NO, after LPS or ConA stimulation, was not detected in either transfectants or CHO-K1 cells. In SOD assay under ConA stimulation, the SOD activity of transfectants was 10 times higher than that of CHO-K1 cells at 6 h after treatment (p < 0.05). The genomic DNA of both the transfectants and control cells began to be fragmented at 6 h after treatment with cyclohexamide. Caspase-3 activity was reduced by transfection with the bovine Prnp (p < 0.05). Conclusively, the viability of transfectants expressing exogenous bovine Prnp was decreased while the capacities for cellular protection against antioxidative stress and apoptosis were increased.
Keyword
MeSH Terms
-
Animals
Apoptosis/physiology
CHO Cells/cytology/enzymology/*physiology
Caspase 3/metabolism
Cattle
Cell Growth Processes/physiology
Cloning, Molecular
Cricetinae
Cricetulus
Encephalopathy, Bovine Spongiform/genetics/*pathology
Formazans
Hydro-Lyases/metabolism
Nitric Oxide/metabolism
Prions/biosynthesis/genetics/*physiology
Superoxide Dismutase/metabolism
Tetrazolium Salts
Transfection
Figure
Reference
-
1. Anderson RM, Donnelly CA, Ferguson NM, Woolhouse MEJ, Watt CJ, Udy HJ, MaWhinney S, Dunstan SP, Southwood TRE, Wilesmith JW, Ryan JBM, Hoinville LJ, Hillerton JE, Austin AR, Wells GAH. Transmission dynamics and epidemiology of BSE in British cattle. Nature. 1996. 382:779–788.
Article2. Bendheim PE, Brown HR, Rudelli RD, Scala LJ, Goller NL, Wen GY, Kascsak RJ, Cashman NR, Bolton DC. Nearly ubiquitous tissue distribution of the scrapie agent precursor protein. Neurology. 1992. 42:149–156.
Article3. Blochberger TC, Cooper C, Peretz D, Tatzelt J, Griffith OH, Baldwin MA, Prusiner SB. Prion protein expression in Chinese hamster ovary cells using a glutamine synthetase selection and amplification system. Protein Eng. 1997. 10:1465–1473.
Article4. Bounhar Y, Zhang Y, Goodyer CG, LeBlanc A. Prion protein protects human neurons against Bax-mediated apoptosis. J Biol Chem. 2001. 276:39145–39149.
Article5. Brown DR, Nicholas RSJ, Canevari L. Lack of prion protein expression results in a neuronal phenotype sensitive to stress. J Neurosci Res. 2002. 67:211–224.
Article6. Caughey B, Race RE, Chesebro B. Detection of prion protein mRNA in normal and scrapie-infected tissues and cell lines. J Gen Virol. 1988. 69:711–716.
Article7. Chen S, Mangé A, Dong L, Lehmann S, Schachner M. Prion protein as trans-interacting partner for neurons is involved in neurite outgrowth and neuronal survival. Mol Cell Neurosci. 2003. 22:227–233.
Article8. Chiarini LB, Freitas AR, Zanata SM, Brentani RR, Martins VR, Linden R. Cellular prion protein transduces neuroprotective signals. EMBO J. 2002. 21:3317–3326.
Article9. Chiesa R, Harris DA. Prion diseases: what is the neurotoxic molecule? Neurobiol Dis. 2001. 8:743–763.
Article10. Florio T, Arena S, Pattarozzi A, Thellung S, Corsaro A, Villa V, Massa A, Diana F, Spoto G, Forcella S, Damonte G, Filocamo M, Benatti U, Schettini G. Basic fibroblast growth factor activates endothelial nitric-oxide synthase in CHO-K1 cells via the activation of cermide synthesis. Mol Pharmacol. 2003. 63:297–310.
Article11. Harris DA. Cellular biology of prion diseases. Clin Microbiol Rev. 1999. 12:429–444.
Article12. Hayashi H, Takata M, Iwamaru Y, Ushiki Y, Mimura KM, Tagawa Y, Shinagawa M, Yokoyama T. Effect of tissue deterioration on postmortem BSE diagnosis by immunobiochemical detection of an abnormal isoform of prion protein. J Vet Med Sci. 2004. 66:515–520.
Article13. Hutter G, Heppner FL, Aguzzi A. No superoxide dismutase activity of cellular prion protein in vivo. Biol Chem. 2003. 384:1279–1285.14. Inoue S, Tanaka M, Horiuchi M, Ishiguro N, Shinagawa M. Characterization of the bovine prion protein gene: the expression requires interaction between the promoter and intron. J Vet Med Sci. 1997. 59:175–183.
Article15. Kang SG, Kang SK, Lee DY, Park YH, Hwang WS, Yoo HS. Cloning, sequencing, and expression of cDNA encoding bovine prion protein. J Microbiol Biotechnol. 2004. 14:417–421.16. Kim BH, Lee HG, Choi JK, Kim JI, Choi EK, Carp RI, Kim YS. The cellular prion protein (PrPC) prevents apoptotic neuronal cell death and mitochondrial dysfunction induced by serum deprivation. Mol Brain Res. 2004. 124:40–50.
Article17. Kim CL, Umetani A, Matsui T, Ishiguro N, Shinagawa M, Horiuchi M. Antigenic characterization of an abnormal isoform of prion protein using a new diverse panel of monoclonal antibodies. Virology. 2004. 320:40–51.
Article18. Korth C, Stierli B, Streit P, Moser M, Schaller O, Fischer R, Schulz-Schaeffer W, Kretzschmar H, Raeber A, Braun U, Ehrensperger F, Hornemann S, Glockshuber R, Riek R, Billeter M, Wuthrich K, Oesch B. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature. 1997. 390:74–77.
Article19. Leclerc E, Peretz D, Ball H, Solforosi L, Legname G, Safar J, Serban A, Prusiner SB, Burton DR, Williamson RA. Conformation of PrPC on the cell surface as probed by antibodies. J Mol Biol. 2003. 326:475–483.20. Lindegren H, Östlund P, Gyllberg H, Bedecs K. Loss of lipopolysaccharide-induced nitric oxide production and inducible nitric oxide synthase expression in scrapie-infected N2a cells. J Neurosci Res. 2003. 71:291–299.
Article21. Lowenstein DH, Butler DA, Westaway D, McKinley MP, DeArmond SJ, Prusiner SB. Three hamster species with different scrapie incubation times and neuropathological features encode distinct prion proteins. Mol Cell Biol. 1990. 10:1153–1163.
Article22. Ma J, Wollmann R, Lindquist S. Neurotoxicity and neurodegeneration when PrP accumulates in the cytosol. Science. 2002. 298:1781–1785.
Article23. Martins VR, Linden R, Prado MAM, Walz R, Sakamoto AC, Izquierdo I, Brentani RR. Cellular prion protein: on the road for functions. FEBS Lett. 2002. 512:25–28.
Article24. Mosnann T. Rapid colorimetric assay for cellular growth and survival. J Immunol Methods. 1983. 65:55–63.25. Nishida N, Tremblay P, Sugimoto T, Shigematsu K, Shirabe S, Petromilli C, Erpel SP, Nakaoke R, Atarashi R, Houtani T, Torchia M, Sakaguchi S, DeArmond SJ, Prusiner SB, Katamine S. A mouse prion protein transgene rescues mice deficient for the prion protein gene from purkinje cell degeneration and demyelination. Lab Invest. 1999. 79:689–697.26. Nunziante M, Gilch S, Schätzl HM. Prion disease: from molecular biology to intervention strategies. Chembiochem. 2003. 4:1268–1284.
Article27. Prusiner SB. Prion disease and the BSE crisis. Science. 1997. 278:245–251.28. Prusiner SB, Fuzi M, Scott M, Serban D, Serban H, Taraboulos A, Gabriel JM, Wells GA, Wilesmith JW, Bradley R. Immunologic and molecular biologic studies of prion proteins in bovine spongiform encephalopathy. J Infect Dis. 1993. 167:602–613.
Article29. Rachidi W, Vilette D, Guiraud P, Arlotto M, Riondel J, Laude H, Lehmann S, Favier A. Expression of prion protein increases cellular copper binding and antioxidant enzyme activities but not copper delivery. J Biol Chem. 2003. 278:9064–9072.
Article30. Roucou X, Gains M, LeBlanc AC. Neuroprotective functions of prion protein. J Neurosci Res. 2004. 75:153–161.
Article31. Sakudo A, Lee DC, Saeki K, Nakamura Y, Inoue K, Matsumoto Y, Itohara S, Onodera T. Impairment of superoxide dismutase activation by N-terminally truncated prion protein (PrP) in PrP-deficient neuronal cell line. Biochem Biophys Res Commun. 2003. 308:660–667.
Article32. Steele AD, Emsley JG, Ozdinler PH, Lindquist S, Macklis JD. Prion protein (PrPC) positively regulates neural precursor proliferation during developmental and adult mammalian neurogenesis. Proc Natl Acad Sci USA. 2006. 103:3416–3421.
Article33. Stewart RS, Harris DA. Mutational analysis of topological determinants in prion protein (PrP) and measurement of transmembrane and cytosolic PrP during prion infection. J Biol Chem. 2003. 278:45960–45968.
Article34. Stuermer CAO, Langhorst MF, Wiechers MF, Legler DF, Von Hanwehr SH, Guse AH, Plattner H. PrPC capping in T cells promotes its association with the lipid raft proteins reggie-1 and reggie-2 and leads to signal transduction. FASEB J. 2004. 18:1731–1733.
Article35. Taylor DR, Hooper NM. The prion protein and lipid rafts. Mol Membr Biol. 2006. 23:89–99.36. Weissmann C, Aguzzi A. Bovine spongiform encephalopathy and early onset variant Creutzfeldt-Jakob disease. Curr Opin Neurobiol. 1997. 7:695–700.
Article37. Wong BS, Pan T, Liu T, Li R, Gambetti P, Sy MS. Differential contribution of superoxide dismutase activity by prion protein in vivo. Biochem Biophys Res Commun. 2000. 273:136–139.
Article38. Yoshimoto J, Iinuma T, Ishiguro N, Horiuchi M, Imamura M, Shinagawa M. Comparative sequence analysis and expression of bovine PrP gene in mouse L-929 cells. Virus Genes. 1992. 6:343–356.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cloning and expression of rat liver type glucose transporter and translocation by insulin in Chinese hamster ovary cells
- Mouse Fyn induces pseudopodium formation in Chinese hamster ovary cells
- A Study on the Induction of Sister-Chromadd Exchanges in Chinese Hamster Ovary Kl Cells by Exposure to Cadmium
- Thyroid Stimulating Antibody Assay with Chinese Hamster Ovary Cells Expressing Human Thyroid Stimulating Hormone (TSH) Receptor; Optimization of Assay Condition
- Production and Characterization of Human CD27lg, CD40fg and CD95lg Fusion Proteins in Chinese Hamster Ovary Cell