Clin Orthop Surg.
2009 Dec;1(4):181-187. 10.4055/cios.2009.1.4.181.
Altered Synthesis of Cartilage-Specific Proteoglycans by Mutant Human Cartilage Oligomeric Matrix Protein
- Affiliations
-
- 1Department of Orthopaedic Surgery, Hallym University Sacred Heart Hospital, Anyang, Korea.
- 2Department of Orthopaedic Surgery, Konyang University Hospital, Daejeon, Korea.
- 3Department of Orthopaedic Surgery, Severance Children's Hospital, Yonsei University College of Medicine, Seoul, Korea. pedhkim@yuhs.ac
- KMID: 999399
- DOI: http://doi.org/10.4055/cios.2009.1.4.181
Abstract
- BACKGROUND
The mechanism by which mutant cartilage oligomeric matrix protein (COMP) induces a pseudoachondroplasia phenotype remains unknown, and the reason why a mutation of a minor protein of the growth plate cartilage causes total disruption of endochondral bone formation has not yet been determined. The current study was performed to investigate the effects of mutated COMP on the synthesis of the cartilage-specific major matrix proteins of Swarm rat chondrosarcoma chondrocytes.
METHODS
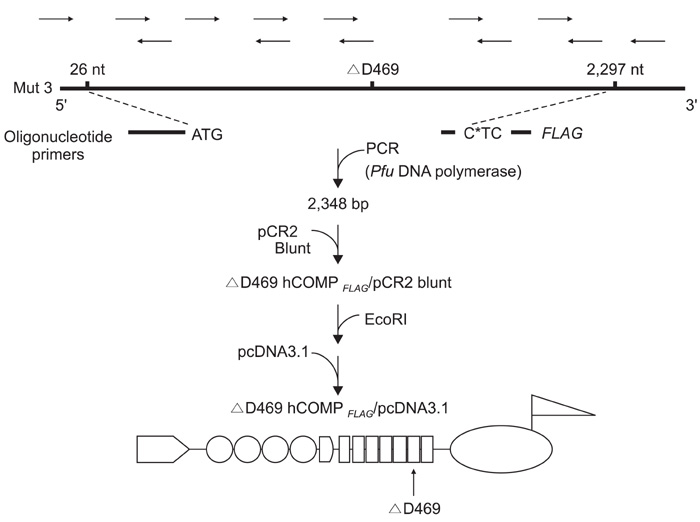
The Swarm rat chondrosarcoma chondrocytes transfected with a chimeric construct, which consisted of a mutant gene of human COMP and an amino acid FLAG tag sequence, were cultured in agarose gel. Formation of extracellular proteoglycan and type-II collagen by the cells was evaluated by immunohistochemical staining and measuring the (35)S-sulfate incorporation.
RESULTS
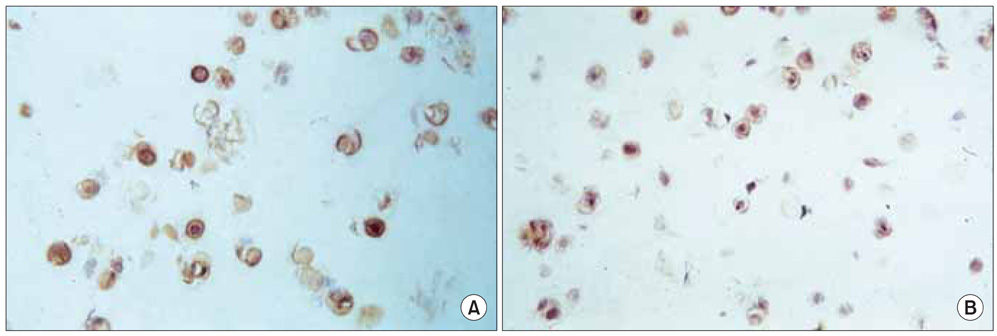
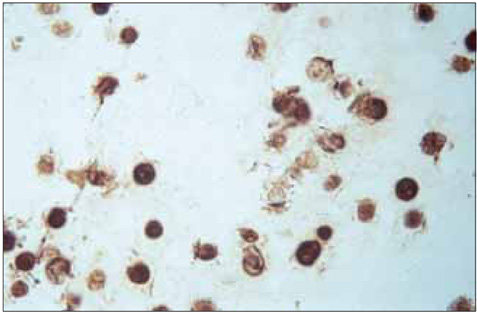
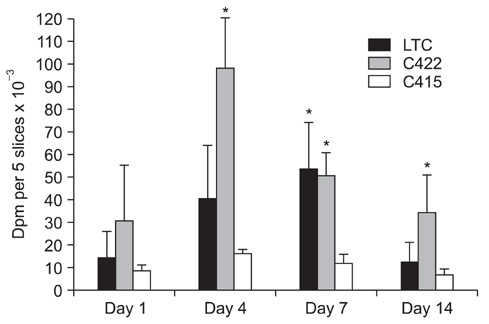
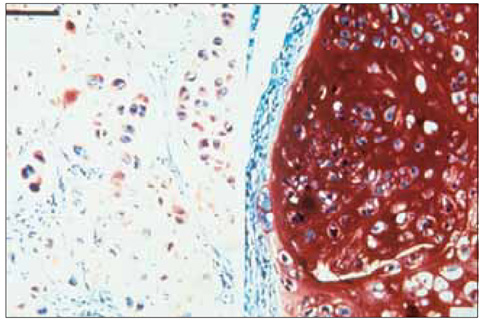
No difference was observed for the detection of type-II collagen among the cell lines expressing mutant COMP and the control cell lines. Histochemical staining of sulfated proteoglycans with safranin-O showed that lesser amounts of proteoglycans were incorporated into the extracellular matrix of the chondrocytes transfected with the mutant gene. (35)S-sulfate incorporation into the cell/matrix fractions demonstrated markedly lower radiolabel incorporation, as compared to that of the control cells.
CONCLUSIONS
Mutation of COMP has an important impact on the processing of proteoglycans, rather than type-II collagen, in the three-dimensional culture of Swarm rat chondrosarcoma chondrocytes.
MeSH Terms
Figure
Cited by 1 articles
-
Pharmacologic treatment of osteoarthritis
Seung-Hoon Baek, Shin-Yoon Kim
J Korean Med Assoc. 2013;56(12):1123-1131. doi: 10.5124/jkma.2013.56.12.1123.
Reference
-
1. Erlebacher A, Filvaroff EH, Gitelman SE, Derynck R. Toward a molecular understanding of skeletal development. Cell. 1995. 80(3):371–378.
Article2. Mundlos S, Olsen BR. Heritable diseases of the skeleton. Part I: Molecular insights into skeletal development-transcription factors and signaling pathways. FASEB J. 1997. 11(2):125–132.
Article3. Stanescu V, Stanescu R, Maroteaux P. Pathogenic mechanisms in osteochondrodysplasias. J Bone Joint Surg Am. 1984. 66(6):817–836.
Article4. Briggs MD, Mortier GR, Cole WG, et al. Diverse mutations in the gene for cartilage oligomeric matrix protein in the pseudoachondroplasia-multiple epiphyseal dysplasia disease spectrum. Am J Hum Genet. 1998. 62(2):311–319.
Article5. Hecht JT, Nelson LD, Crowder E, et al. Mutations in exon 17B of cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasia. Nat Genet. 1995. 10(3):325–329.
Article6. Delot E, Brodie SG, King LM, Wilcox WR, Cohn DH. Physiological and pathological secretion of cartilage oligomeric matrix protein by cells in culture. J Biol Chem. 1998. 273(41):26692–26697.
Article7. Maynard JA, Cooper RR, Ponseti IV. A unique rough surfaced endoplasmic reticulum inclusion in pseudoachondroplasia. Lab Invest. 1972. 26(1):40–44.8. Hecht JT, Deere M, Putnam E, et al. Characterization of cartilage oligomeric matrix protein (COMP) in human normal and pseudoachondroplasia musculoskeletal tissues. Matrix Biol. 1998. 17(4):269–278.
Article9. Chen H, Deere M, Hecht JT, Lawler J. Cartilage oligomeric matrix protein is a calcium-binding protein, and a mutation in its type 3 repeats causes conformational changes. J Biol Chem. 2000. 275(34):26538–26544.
Article10. Maddox BK, Mokashi A, Keene DR, Bachinger HP. A cartilage oligomeric matrix protein mutation associated with pseudoachondroplasia changes the structural and functional properties of the type 3 domain. J Biol Chem. 2000. 275(15):11412–11417.
Article11. Fellini SA, Kimura JH, Hascall VC. Polydispersity of proteoglycans synthesized by chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1981. 256(15):7883–7889.
Article12. Oldberg A, Antonsson P, Lindblom K, Heinegard D. COMP (cartilage oligomeric matrix protein) is structurally related to the thrombospondins. J Biol Chem. 1992. 267(31):22346–22350.
Article13. DiCesare P, Hauser N, Lehman D, Pasumarti S, Paulsson M. Cartilage oligomeric matrix protein (COMP) is an abundant component of tendon. FEBS Lett. 1994. 354(2):237–240.
Article14. Hecht JT, Montufar-Solis D, Decker G, Lawler J, Daniels K, Duke PJ. Retention of cartilage oligomeric matrix protein (COMP) and cell death in redifferentiated pseudoachondroplasia chondrocytes. Matrix Biol. 1998. 17(8-9):625–633.
Article15. Stevens JW, Rapp TB, Martin JA, Maynard JA, Vertel BA, Hecht JT. Stable transfection of chondrocytes with mutant COMP. Trans Orthop Res Soc. 1998. 23:103.16. Morgelin M, Heinegard D, Engel J, Paulsson M. Electron microscopy of native cartilage oligomeric matrix protein purified from the Swarm rat chondrosarcoma reveals a five-armed structure. J Biol Chem. 1992. 267(9):6137–6141.
Article17. Newton G, Weremowicz S, Morton CC, et al. Characterization of human and mouse cartilage oligomeric matrix protein. Genomics. 1994. 24(3):435–439.
Article18. Briggs MD, Hoffman SM, King LM, et al. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat Genet. 1995. 10(3):330–336.
Article19. Cooper RR, Ponseti IV, Maynard JA. Pseudoachondroplastic dwarfisma: a rough-surfaced endoplasmic reticulum storage disorder. J Bone Joint Surg Am. 1973. 55(3):475–484.20. Stanescu V, Maroteaux P, Stanescu R. The biochemical defect of pseudoachondroplasia. Eur J Pediatr. 1982. 138(3):221–225.
Article21. Breur GJ, Farnum CE, Padgett GA, Wilsman NJ. Cellular basis of decreased rate of longitudinal growth of bone in pseudoachondroplastic dogs. J Bone Joint Surg Am. 1992. 74(4):516–528.
Article22. Stevens JW. Pseudoachondroplastic dysplasia: an Iowa review from human to mouse. Iowa Orthop J. 1999. 19:53–65.23. Kim HW, Han CD. An overview of cartilage tissue engineering. Yonsei Med J. 2000. 41(6):766–773.
Article24. Bornstein P. Thrombospondins: structure and regulation of expression. FASEB J. 1992. 6(14):3290–3299.
Article25. Kuznetsov G, Chen LB, Nigam SK. Multiple molecular chaperones complex with misfolded large oligomeric glycoproteins in the endoplasmic reticulum. J Biol Chem. 1997. 272(5):3057–3063.
Article26. Maddox BK, Keene DR, Sakai LY, et al. The fate of cartilage oligomeric matrix protein is determined by the cell type in the case of a novel mutation in pseudoachondroplasia. J Biol Chem. 1997. 272(49):30993–30997.
Article27. Thur J, Rosenberg K, Nitsche DP, et al. Mutations in cartilage oligomeric matrix protein causing pseudoachondroplasia and multiple epiphyseal dysplasia affect binding of calcium and collagen I, II, and IX. J Biol Chem. 2001. 276(9):6083–6092.
Article28. Dinser R, Zaucke F, Kreppel F, et al. Pseudoachondroplasia is caused through both intra- and extracellular pathogenic pathways. J Clin Invest. 2002. 110(4):505–513.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Mutant Cartilage Oligomeric Matrix Protein on the Synthesis of Extracellular Matrix in the Swarm Rat Chondrosarcoma Cell Line
- Intermittent Negative Hydrostatic Pressure and Chondrocyte Metabolism
- Viability of Refrigerated Human Articular Cartilage
- Degree of degeneration and Chondroitinase ABC treatment of human articular cartilage affect the adhesion of transplanted chondrocyte
- Expression Of Igf-1 And Tgf-beta In Rheumatoid Arthritis And O~teoarthritis