Korean J Radiol.
2002 Sep;3(3):180-188. 10.3348/kjr.2002.3.3.180.
Metabolic Alterations in Parkinson's Disease after Thalamotomy, as Revealed by 1H MR Spectroscopy
- Affiliations
-
- 1Department of Biomedical Engineering, Kangnam St. Mary's Hospital College of Medicine, The Catholic University of Korea, Seoul, Korea. bychoe@catholic.ac.kr
- 2Department of Neurosurgery, Kangnam St. Mary's Hospital College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Radiology, Kangnam St. Mary's Hospital College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 754060
- DOI: http://doi.org/10.3348/kjr.2002.3.3.180
Abstract
OBJECTIVE
To determine, using proton magnetic resonance spectroscopy (1H MRS) whether thalamotomy in patients with Parkinson's disease gives rise to significant changes in regional brain metabolism.
MATERIALS AND METHODS
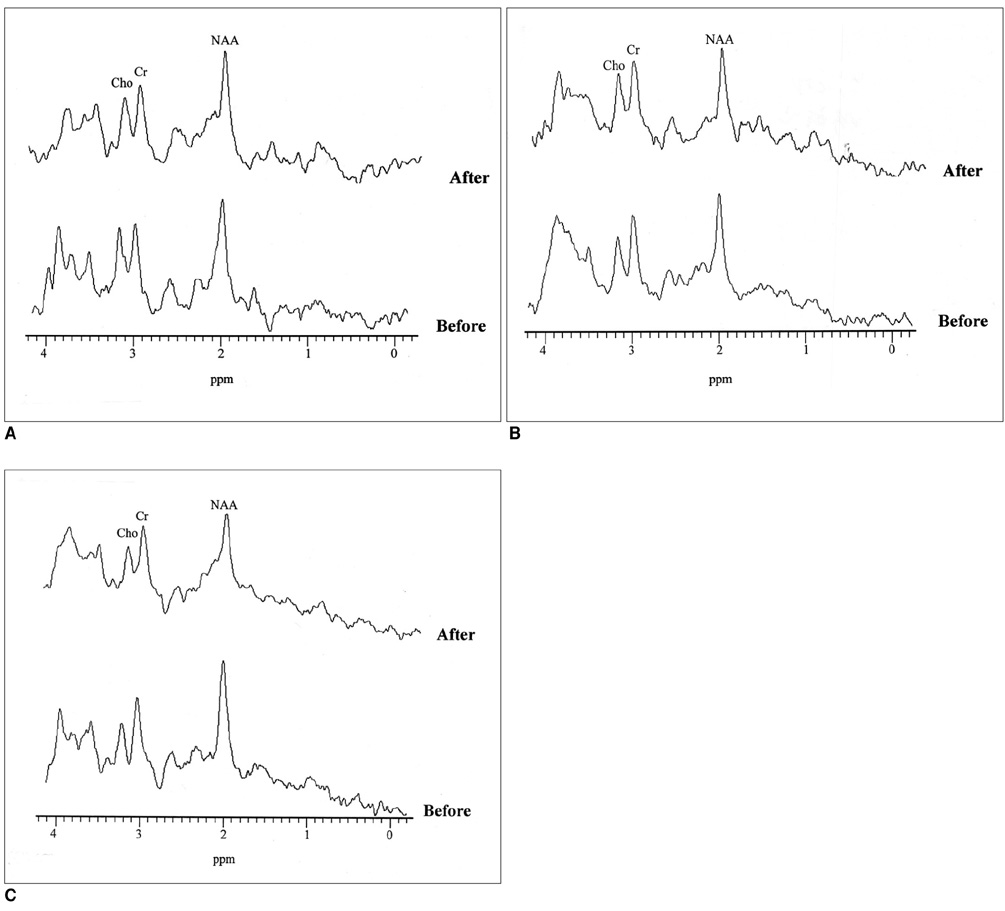
Fifteen patients each underwent stereotactic thalamotomy for the control of medically refractory parkinsonian tremor. Single-voxel 1H MRS was performed on a 1.5T unit using a STEAM sequence (TR/TM/TE, 2000/14/20 msec), and spectra were obtained from substantia nigra, thalamus and putamen areas, with volumes of interest of 7-8ml, before and after thalamotomy. NAA/Cho, NAA/Cr and Cho/Cr metabolite ratios were calculated from relative peak area measurements, and any changes were recorded and assessed.
RESULTS
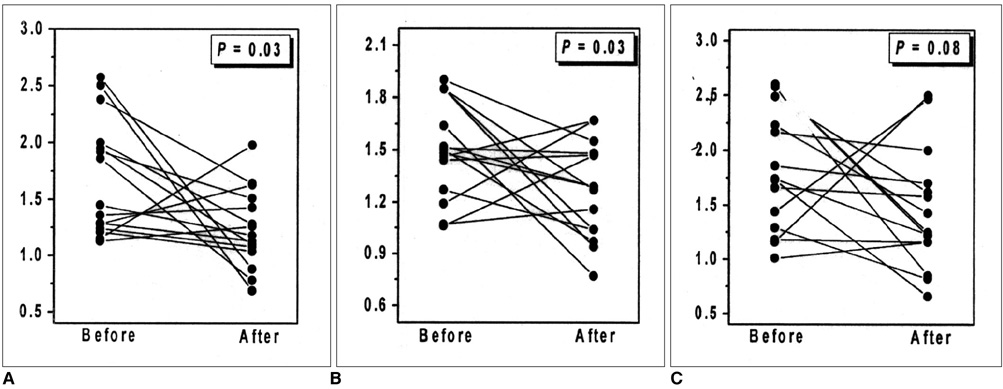
In the substantia nigra and thalamus, NAA/Cho ratios were generally low. In the substantia nigra of 80% of patients (12/15) who showed clinical improvement, decreased NAA/Cho ratios were observed in selected voxels after thalamic surgery (p < 0.05). In the thalamus of 67% of such patients (10/15), significant decreases were also noted (p < 0.05).
CONCLUSION
Our results suggest that the NAA/Cho ratio may be a valuable criterion for the evaluation of Parkinson's disease patients who show clinical improvement following surgery. By highlighting variations in this ratio, 1H MRS may help lead to a better understanding of the pathophysiologic processes occurring in those with Parkinson's disease.
Keyword
MeSH Terms
-
Adult
Aged
Aspartic Acid/*analogs & derivatives/metabolism
Brain/*metabolism/pathology
Choline/metabolism
Female
Human
Magnetic Resonance Imaging
Magnetic Resonance Spectroscopy
Male
Middle Age
Parkinson Disease/*metabolism/pathology/*surgery
Protons
Putamen/metabolism/pathology
Substantia Nigra/metabolism/pathology
Thalamus/*metabolism/pathology/*surgery
Figure
Reference
-
1. Barbeau A. Six years of high-level levodopa therapy in severely akinetic parkinsonian patients. Arch Neurol. 1976. 33:333–338.2. Boshes B, Arbit J, Dolkart M, et al. The sixth year after L-dopa: quality of life and insights derived. Trans Am Neurol Assoc. 1975. 100:166–168.3. Delaney P, Fermaglich J. Parkinsonism and levodopa: a five-year experience. J Clin Pharmacol. 1976. 16:652–659.4. Jankovic J, Cardoso F, Grossman RG, Hamilton WJ. Outcome after stereotactic thalamotomy for parkinsonian, essential, and other types of tremor. Neurosurgery. 1995. 37:680–686.5. Lang AE, Lozano AM, Montgomery E, Duff J, Tasker R, Hutchinson W. Posteroventral medial pallidotomy in advanced Parkinson's disease. N Engl J Med. 1997. 337:1036–1042.6. Samuel M, Ceballos-Baumann AO, Turjanski N, et al. Pallidotomy in Parkinson's disease increases supplementary motor area and prefrontal activation during performance of volitional movements on H215O PET study. Brain. 1997. 120:1301–1313.7. Davis KD, Taub E, Houle S, et al. Globus pallidus stimulation activates the cortical motor system during allevation of parkinsonian symptoms. Nat Med. 1997. 3:671–674.8. Limousin P, Greene J, Pollak D, Rothwell J, Benabid AL, Frakowiak R. Changes in cerebral activity pattern due to subthalamic nucleus or internal pallidum stimulation in Parkinson's disease. Ann Neurol. 1997. 42:283–291.9. Dogali M, Fazzini E, Kolodny E, et al. Stereotactic ventral pallidotomy for Parkinson's disease. Neurology. 1995. 45:753–761.10. Burchiel KJ. Thalamotomy for movement disorders. Neurosurg Clin N Am. 1995. 6:55–71.11. Fox MW, Ahlskog JE, Kelly PJ. Stereotactic ventrolateralis thalamotomy for medically tremor in post-levodopa era Parkinson's disease. Neurosurgery. 1995. 36:1118–1127.12. Holshouser BA, Komu M, Moller HE, et al. Localized proton NMR spectroscopy in the striatum of patients with idiopathic Parkinson's disease: a multicentre pilot study. Magn Reson Med. 1995. 33:589–594.13. Ellis CM, Lemmens G, Williams SC, et al. Changes in putamen N-acetylaspartate and choline ratios in untreated and levodopa-treated Parkinson's disease: a proton magnetic resonance spectroscopy study. Neurology. 1997. 49:438–444.14. Choe BY, Park JW, Lee KS, et al. Neuronal laterality in Parkinson's disease with unilateral symptom by in-vivo 1H MR spectroscopy. Invest Radiol. 1998. 33:450–455.15. Bottomley PA. Spatial localization in NMR spectroscopy in-vivo. Ann NY Acad Sci. 1987. 508:333–348.16. Hughes A, Daniel S, Kilford L, Lees A. Accuracy of clinical diagnosis of idiopathic Parkinsion's disease: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992. 55:181–184.17. Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent development in Parkinson's disease. 1987. Vol 2. Florham Park, NJ: Macmillan Health Care Information;153–163.18. The Parkinson Study Group. Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson's disease. N Engl J Med. 1999. 340:757–763.19. Frahm J, Merbold KD, Hanicke W. Localized proton spectroscopy using stimulated echoes. J Magn Reson. 1987. 72:502–508.20. Moonen CTW, van Zijl PCM. Highly effective water suppression for in-vivo proton NMR spectroscopy (DRY STEAM). J Magn Reson. 1990. 88:28–41.21. Hasse A, Frahm J, Hanicke W, Mattaei D. 1H NMR chemical shift selective (CHESS) imaging. Phys Med Biol. 1985. 30:341.22. Doddrell DM, Galloway G, Brooks W, et al. Water signal elimination in-vivo using suppression by mistimed echo and repetitive gradient episodes. J Magn Reson. 1986. 70:176–180.23. Kreis R, Farrow N, Ross BD. Localized 1H NMR spectroscopy in patients with chronic hepatic encephalopathy: analysis of change in cerebral glutamate, choline, and inositols. NMR in Biomed. 1994. 4:109–116.24. Drayer BP, Olanow W, Burger P, et al. Parkinson-plus syndrome: diagnosis using high-field MR imaging of brain iron. Radiology. 1989. 159:493–498.25. Stern MB, Braffman BH, Skolnick BE, et al. Magnetic resonance imaging in Parkinson's disease and Parkinsonian syndromes. Neurology. 1989. 39:1524–1526.26. Benabid AL, Pollak P, Gao D, et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996. 84:203–214.27. Goldman MS, Ahlskog E, Kelly P. The symptomatic and functional outcome of stereotactic thalamotomy for medically intractable essential tremor. J Neurosurg. 1992. 76:924–928.28. Jankovic J, Cardoso F, Grossman R, et al. Outcome after stereotactic thalamotomy for Parkinsonian, essential, and other types of tremor. Neurosurgery. 1995. 37:680–687.29. Nagaseki Y, Shibazaki T, Hirai T, et al. Long-term follow-up results of selective VIM-thalamotomy. J Neurosurg. 1986. 65:296–302.30. Lenz FA, Normand SL, Kwan HC, et al. Statistical prediction of the optimal site for thalamotomy in parkinsonian tremor. Mov Disord. 1995. 10:318–328.31. Birken DL, Oldendorf WH. N-acetyl-aspartate acid: a literature review of a compound prominent in 1H NMR spectroscopic studies of the brain. Neurosci Biobehav Rev. 1989. 13:23–31.32. Miller BL. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed. 1991. 4:47–52.33. Bruhn H, Frahm J, Gyngell ML, et al. Noninvasive differentiation of tumors with use of localized 1H MR spectroscopy in vivo: initial experience in patients with cerebral tumors. Radiology. 1989. 172:541–548.34. Langkowski JH, Wieland J, Bomsdorf H, et al. Pre-operative localized in-vivo proton spectroscopy in cerebral tumors at 4.0 Tesla-first results. Magn Reson Imaging. 1989. 7:547–555.35. Gill SS, Thomas DG, Van Bruggen N, et al. Proton MR spectroscopy of intracranial tumors: in-vivo and in-vitro studies. J Comput Assist Tomogr. 1990. 14:497–504.36. Gill SS, Thomas DG, Thomas DG, et al. Brain metabolites as 1H NMR markers of neuronal and glial disorders. NMR Biomed. 1989. 2:196–200.37. Koller KJ, Zaczek R, Coyle JT. N-acetyl-aspartyl-glutamate: regional levels in rat brain and the effects of brain lesions as determined by a new HPLC method. J Neurochem. 1984. 43:1136–1142.38. Bruhn H, Frahm J, Gyngell ML, Merboldt KD, Hanicke W, Sauter R. Cerebral metabolism in man after acute stroke: new observations using localized proton NMR spectroscopy. Magn Reson Med. 1989. 9:126–131.39. Menon DK, Baudouin CJ, Tomlinson D, Hoyle C. Proton MR spectroscopy and imaging of the brain in AIDS: evidence of neuronal loss in regions that appear normal with imaging. J Comput Assist Tomogr. 1990. 14:882–885.40. Van Hecke P, Marchal G, Johannik K, et al. Human brain proton localized NMR spectroscopy in multiple sclerosis. Magn Reson Med. 1991. 18:199–206.41. Matthews PM, Francis G, Antel J, Arnold DL. Proton magnetic resonance spectroscopy for metabolic characterization of plaques in multiple sclerosis. Neurology. 1991. 41:1251–1256.42. Kreis R, Ross BD. Cerebral metabolic disturbances in patients with subacute and chronic diabetes mellitus: detection with proton MR spectroscopy. Radiology. 1992. 184:123–130.43. Ende GR, Kaxer KD, Knowlton RC, et al. Temporal lobe epilepsy: bilateral hippocampal metabolic changes revealed at proton MR spectroscopic imaging. Radiology. 1997. 202(3):809–817.44. Chang KH, Kim HD, Park SW, et al. Usefulness of single voxel proton MR spectroscopy in the evaluation of hippocampal sclerosis. Korean J Radiol. 2000. 1:25–32.45. Lu D, Pavlakis SG, Frank Y, et al. Proton MR spectroscopy of the basal ganglia in healthy children and children with AIDS. Radiology. 1996. 199:423–428.46. Shonk TK, Moats RA, Gifford P, et al. Probable Alzheimer disease: diagnosis with proton MR spectroscopy. Radiology. 1995. 195:65–72.47. Connelly A, Jackson GD, Duncan JS, King MD, Gadian DG. Magnetic resonance spectroscopy in temporal lobe epilepsy. Neurology. 1994. 44:1411–1417.48. Matthews PM, Andermann F, Arnold DL. A proton magnetic resonance spectroscopy study of focal epilepsy in humans. Neurology. 1990. 40:985–989.49. Brown BC, Block RE, Sanchez-Ramos J, et al. Proton MR spectroscopy of the brain in vivo in 14 patients with Parkinson's disease. Am J Neuroradiol. 1995. 16:61–68.50. Beal MF. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illness? Ann Neurol. 1992. 31:119–130.51. Federico F, Simone IL, Lucivero V, et al. Localized proton NMR spectroscopy in Parkinson's disease and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 1997. 62:239–242.52. Clarke CE, Lowy M, Horsman A. Unchanged basal ganglia N-acetylaspartate and glutamate in idiopathic Parkinson's disease measured by proton magnetic resonance spectroscopy. Mov Disord. 1997. 12:297–301.53. Davie CA, Wenning GK, Barker GJ, et al. Differentiation of multiple system atrophy from idiopathic Parkinson's disease using proton magnetic resonance spectroscopy. Ann Neurol. 1995. 37:204–210.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retraction: Metabolic Alterations in Parkinson's Disease after Thalamotomy, as Revealed by 1H MR Spectroscopy
- In vivo H MR spectroscopy of human brain in six normal volunteers
- Localized, water-suppressed in vivo H MR spectroscopy of human brain tumors: Preliminary results
- H MR Spectroscopy on Parkinson's-like Syndrome Induced by Manganese Intoxication: A Case Report
- Localized 1H-MR Spectroscopy in Moyamoya Disease before and after Revascularization Surgery