Korean J Radiol.
2003 Jun;4(2):71-78. 10.3348/kjr.2003.4.2.71.
Localized 1H-MR Spectroscopy in Moyamoya Disease before and after Revascularization Surgery
- Affiliations
-
- 1Department of Diagnostic Radiology, College of Medicine, Ewha Womans University Hospital, Seoul Korea. soomee@mm.ewha.ac.kr
- 2NMR Laboratory, Asan Institute for Life Sciences.
- 3Department of Diagnostic Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul Korea.
- 4Department of Neurosurgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul Korea.
- KMID: 1118808
- DOI: http://doi.org/10.3348/kjr.2003.4.2.71
Abstract
OBJECTIVE
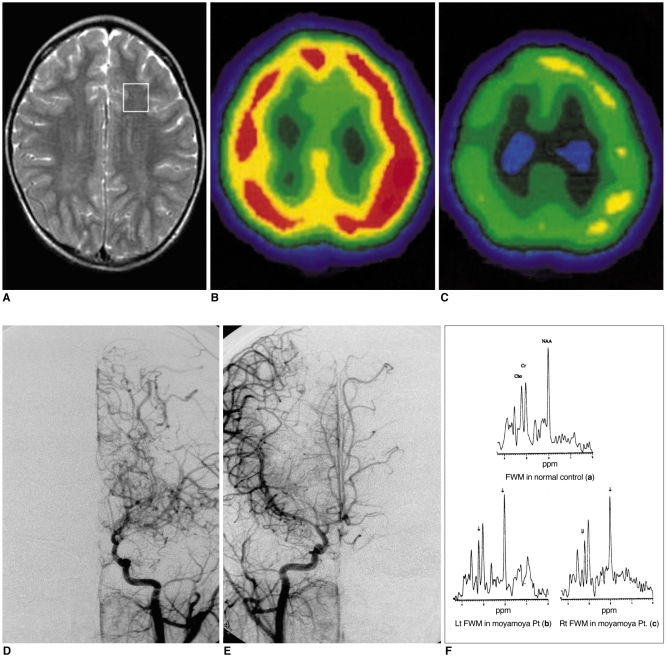
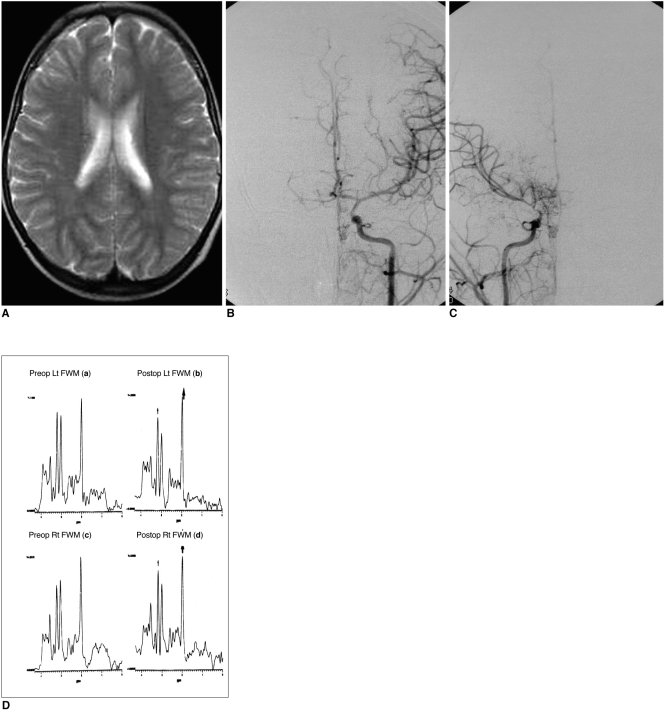
To evaluate, using localized proton magnetic resonance spectroscopy (1H-MRS), the cerebral metabolic change apparent after revascularization surgery in patients with moyamoya disease. MATERIALS AND METHODS: Sixteen children with moyamoya disease and eight age-matched normal controls underwent MR imaging, MR angiography, conventional angiography, and 99mTc- ECD SPECT. Frontal white matter and the basal ganglia of both hemispheres were subjected to localized 1H-MRS, and after revascularization surgery, four patients underwent follow-up 1H-MRS. RESULTS: Decreased NAA/Cr ratios (1.35+/-0.14 in patients vs. 1.55+/-0.24 in controls) and Cho/Cr ratios (0.96+/-0.13 in patients vs. 1.10+/-0.11 in controls) were observed in frontal white matter. After revascularization surgery, NAA/Cr and Cho/Cr ratios in this region increased. In the basal ganglia, there is no abnormal metabolic ratios. CONCLUSION: Localized 1H-MRS revealed abnormal metabolic change in both hemispheres of children with moyamoya disease. Because of its non-invasive nature, 1H-MRS is potentially useful for the preoperative evaluation of metabolic abnormalities and their postoperative monitoring.
Figure
Reference
-
1. Kudoh T. Juvenile occlusion of the circle of Willis. Clin Neurol. 1965; 5:607.2. Kuwabara Y, Ichiya Y, Otsuka M, et al. Cerebral hemodynamic change in the child and the adult with moyamoya disease. Stroke. 1990; 21:272–277. PMID: 2305403.
Article3. Suzuki J, Takaku A. Cerebrovascular "moyamoya" disease: disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969; 20:288–299. PMID: 5775283.4. Halbach VV, Barkovich AJ. Anomalies of cerebral vasculature: Pediatric neuroimaging. 1995. 2nd ed. New York: Raven Press;p. 619–653.5. Kinugasa K, Mandai S, Kamata I, Sugiu K, Ohmoto T. Surgical treatment of moyamoya disease: operative technique for encephalo-duro-arterio-myo-synangiosis, its follow-up, clinical results, and angiograms. Neurosurgery. 1993; 32:527–531. PMID: 8474642.6. Ishikawa T, Houkin K, Kamiyama H, Abe H. Effects of surgical revascularization on outcome of patients with pediatric moyamoya disease. Stroke. 1997; 28:1170–1173. PMID: 9183345.
Article7. Matsushima Y, Fukai N, Tanaka K, et al. A new surgical treatment of moyamoya disease in children: a preliminary report. Surg Neurol. 1987; 15:313–320. PMID: 7245020.
Article8. Ross BD, Michaelis T. Clinical applications of magnetic resonance spectroscopy. Magn Reson Q. 1994; 10:191–247. PMID: 7873353.9. Kimura H, Fujii Y, Itoh S, et al. Metabolic alterations in neonate and infant brain during development : evaluation with proton MR spectroscopy. Radiology. 1995; 194:483–489. PMID: 7529934.10. Lee JH, Arcinue E, Ross BD. Organic osmolytes in the brain of an infant with hypernatremia. N Engl J Med. 1994; 331:439–442. PMID: 8035840.
Article11. Van der Grond J, Balm R, Kappelle L, Eikelboom BC, Mali WP. Cerebral metabolism of patients with stenosis or occlusion of the internal carotid artery: A 1H-MR spectroscopic imaging study. Stroke. 1995; 26:822–828. PMID: 7740574.12. Kreis R, Ross BD, Farrow NA, Ackerman Z. Metabolic disorders of the brain in chronic hepatic encephalopathy detected with H-1 MR spectroscopy. Radiology. 1992; 182:19–27. PMID: 1345760.
Article13. Bizzi A, Movsas B, Tedeschi G, et al. Response of non-Hodgkin lymphoma to radiation therapy: early and long-term assessment with H-1 MR spectroscopic imaging. Radiology. 1995; 194:271–276. PMID: 7997566.
Article14. Rajanayagam V, Grad J, Krivit W, et al. Proton MR spectroscopy of childhood adrenoleukodystrophy. AJNR Am J Neuroradiol. 1996; 17:1013–1024. PMID: 8791909.15. Duijin JH, Matson GB, Maudsley AA, Hugg JW, Weiner MW. Human brain infarction: Proton MR spectroscopy. Radiology. 1992; 183:711–718. PMID: 1584925.
Article16. Lanfermann H, Kugel H, Heindel W, Herholz K, Heiss W-D, Lackner K. Metabolic changes in acute and subacute cerebral infarctions: findings at proton MR spectroscopic imaging. Radiology. 1995; 196:203–210. PMID: 7784568.
Article17. Van der Grond J, Ramos LMP, Eikelboom BC, Mali WP. Cerebral metabolic differences between the severe and critical hypoperfused brain. Neurology. 1996; 47:399–404. PMID: 8757011.
Article18. Takemichi TK, Prohovnik I, Mohr JP, Correll JW, Quest DO, Jarvis L. Reduced hypercapnic vasoreactivity in moyamoya disease. Neurology. 1988; 38:1575–1581. PMID: 3419602.
Article19. Taki W, Yonekawa Y, Kobayashi A, et al. Cerebral circulation and metabolism in adult's moyamoya disease: PET study. Acta Neurochir (Wien). 1989; 100:150–154. PMID: 2589122.20. Ogawa A, Yoshimoto T, Suzuki J, Sakurai Y. Cerebral blood flow in moyamoya disease, part 1: correlation with age and regional distribution. Acta Neurochir (Wien). 1990; 105:30–34. PMID: 2239376.21. Ogawa A, Nakamura N, Yoshimoto T, Suzuki J. Cerebral blood flow in moyamoya disease, part 2: autoregulation and CO2 response. Acta Neurochir (Wien). 1990; 105:107–111. PMID: 2125802.22. Sato H, Sato N, Tamuki N, Matsumoto S. Chronic low-perfusion state in children with moyamoya disease following revascularization. Childs Nerv Syst. 1990; 6:166–171. PMID: 2357714.
Article23. Ohashi K, Fernandez-Ulloa M, Hall LC. SPECT, magnetic resonance and angiographic features in a moyamoya patient before and after external-to-internal carotid artery bypass. J Nucl Med. 1992; 33:1692–1695. PMID: 1517845.24. Inoue Y, Momose T, Machida K, Honda N, Tsutsumi K. Cerebral vasodilatory capacity mapping using technetium-99m-DTPA-HAS SPECT and acetazolamide in moyamoya disease. J Nucl Med. 1993; 34:1984–1986. PMID: 8229245.25. Rutgers DR, Klijn CJ, Kappelle LJ, van der Grond J. Cerebral metabolic changes in patients with symptomatic occlusion of the internal carotid artery: a longitudinal 1H magnetic resonance spectroscopy study. J Magn Reson Imaging. 2000; 11:279–286. PMID: 10739559.26. Janson C, McPhee S, Bilaniuk L, et al. Clinical protocol gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum Gene Ther. 2002; 13:1391–1412. PMID: 12162821.27. Hashimoto T, Tayama M, Miyazaki M, et al. Developmental brain changes investigated with proton magnetic resonance spectroscopy. Dev Med Child Neurol. 1995; 37:398–405. PMID: 7768339.
Article28. Shimizu H, Shirane R, Fujiware S, Takahashi A, Yoshimoto T. Proton magnetic resonance spectroscopy in children with moyamoya disease. Clin Neurol Neurosurg. 1997; 99:S64–S67. PMID: 9409409.
Article29. Touho H, Karasawa J, Ohnishi H. Preoperative and postoperative evaluation of cerebral perfusion and vasodilatory capacity with 99mTc-HMPAO SPECT and acetazolamide in childhood moyamoya disease. Stroke. 1996; 27:282–289. PMID: 8571424.30. Hoshi H, Ohnishi T, Jinnouchi S, et al. Cerebral blood flow study in patients with moyamoya disease evaluated by IMP SPECT. J Nucl Med. 1994; 35:44–50. PMID: 8271059.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroimaging Diagnosis and Treatment of Moyamoya Disease
- Localized, water-suppressed in vivo H MR spectroscopy of human brain tumors: Preliminary results
- In vivo H MR spectroscopy of human brain in six normal volunteers
- Metabolic Changes after Revascularization in a Patient with Innominate Artery Occlusion by Localized in vivo Proton Magnetic Resonance Spectroscopy
- Usefulness of 1H Magnetic Resonance Spectroscopy for Evaluation of Cognitive Impairment after Subarachnoid Hemorrhage Caused by Rupture of Middle Cerebral Artery Aneurysm