Korean J Radiol.
2004 Jun;5(2):87-95. 10.3348/kjr.2004.5.2.87.
Functional Neuroanatomy in Depressed Patients with Sexual Dysfunction: Blood Oxygenation Level Dependent Functional MR Imaging
- Affiliations
-
- 1Department of Psychiatry, Chonnam National University Hospital, Kwangju, Korea. jcyang@chonnam.ac.kr
- KMID: 753987
- DOI: http://doi.org/10.3348/kjr.2004.5.2.87
Abstract
OBJECTIVE
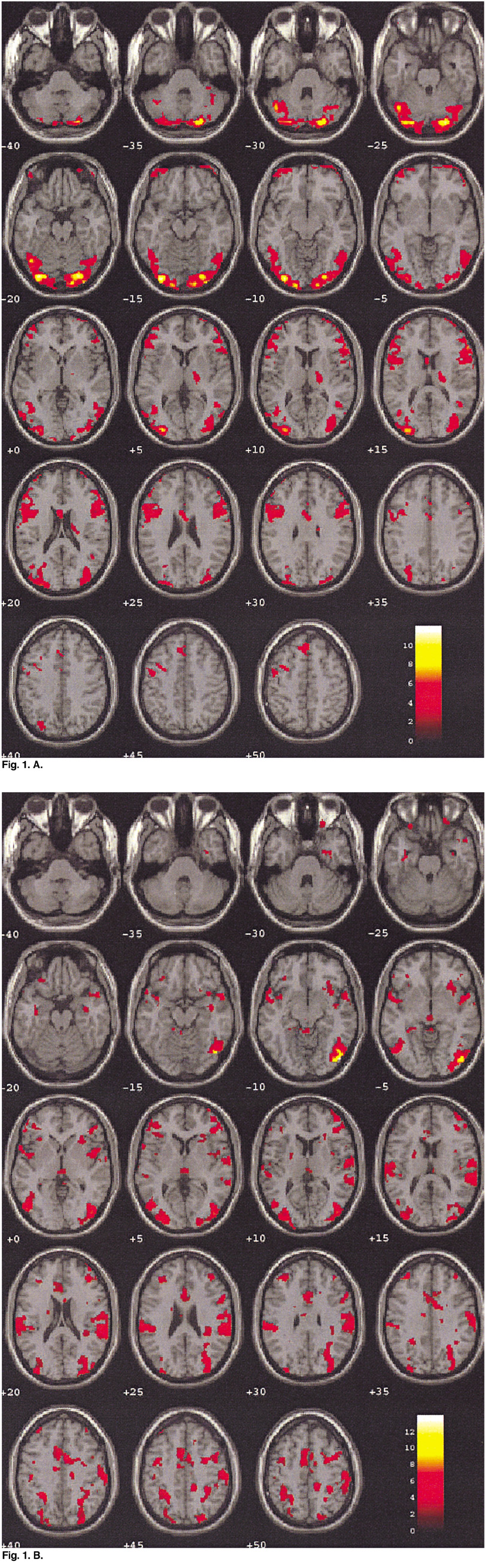
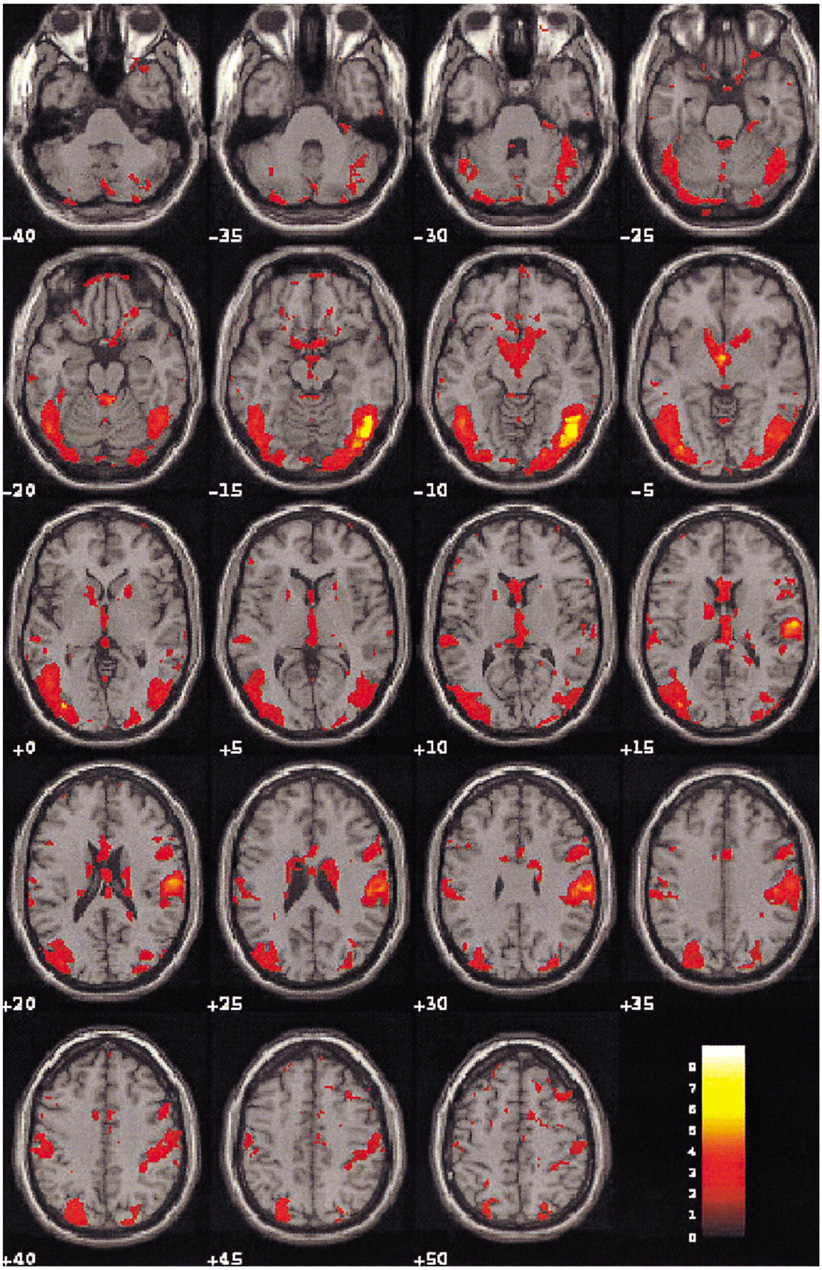
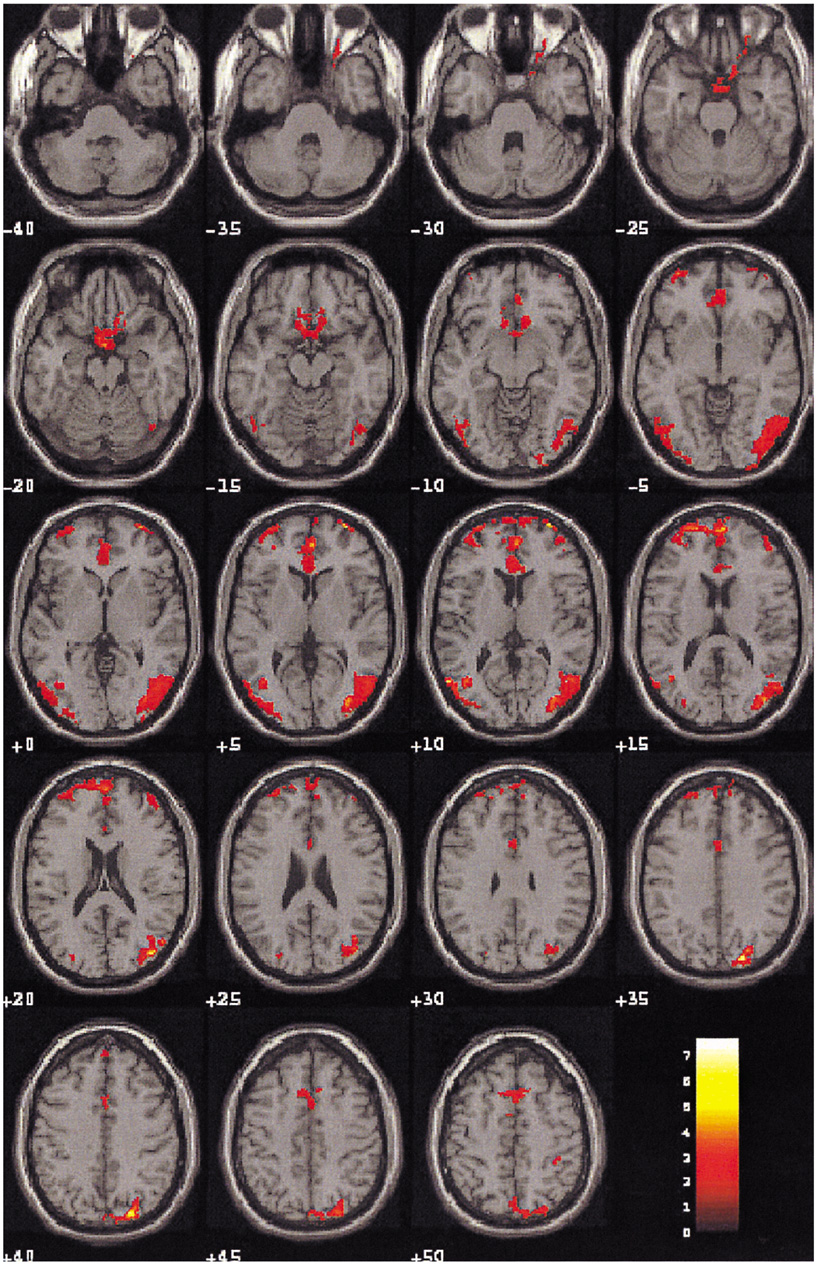
To demonstrate the functional neuroanatomy associated with sexual arousal visually evoked in depressed males who have underlying sexual dysfunction using Blood Oxygenation Level Dependent-based fMRI. MATERIALS AND METHODS: Ten healthy volunteers (age range 21-55: mean 32.5 years), and 10 depressed subjects (age range 23-51: mean 34.4 years, mean Beck Depression Inventory score of 39.6+/-5.9, mean Hamilton Rating Scale Depression (HAMD) -17 score of 33.5+/-6.0) with sexual arousal dysfunction viewed erotic and neutral video films during functional magnetic resonance imaging (fMRI) with 1.5 T MR scanner (GE Signa Horizon). The fMRI data were obtained from 7 oblique planes using gradient-echo EPI (flip angle/TR/TE= 90 degrees/6000 ms/50 ms). The visual stimulation paradigm began with 60 sec of black screen, 150 sec of neutral stimulation with a documentary video film, 30 sec of black screen, 150 sec of sexual stimulation with an erotic video film followed by 30 sec of black screen. The brain activation maps and their quantification were analyzed by SPM99 program. RESULTS: There was a significant difference of brain activation between two groups during visual sexual stimulation. In depressed subjects, the level of activation during the visually evoked sexual arousal was significantly less than that of healthy volunteers, especially in the cerebrocortical areas of the hypothalamus, thalamus, caudate nucleus, and inferior and superior temporal gyri. On the other hand, the cerebral activation patterns during the neutral condition in both groups showed no significant differences (p < 0.01). CONCLUSION: This study is the first demonstration of the functional neuroanatomy of the brain associated with sexual dysfunction in depressed patients using fMRI. In order to validate our physiological neuroscience results, further studies that would include patients with other disorders and sexual dysfunction, and depressed patients without sexual dysfunction and their treatment response are needed.
Keyword
MeSH Terms
Figure
Reference
-
1. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960. 12:56–62.2. Nelson JC, Charney DS. The symptoms of major depressive illness. Am J Psychiatry. 1981. 138:1–12.3. Casper RC, Redmond DE, Katz MM, Schaffer CB, Davis JM, Koslow SH. Somatic symptoms in primary affective disorder. Arch Gen Psychiatry. 1985. 42:1098–1104.4. Matthew RJ, Weinman ML. Sexual dysfunctions in depression. Arch Sex Behav. 1982. 11:323–328.5. Tamburello A, Seppecher MF. Gemme R, Wheeler CC, editors. The effects of depression on sexual behavior: preliminary results of research. Progress in Sexology. 1977. New York, NY: Plenum Press;107–128.6. Thase ME, Reynolds CF, Jennings JR, et al. Nocturnal penile tumescence is diminished in depressed men. Biol Psychiatry. 1988. 24:33–46.7. George MS, Ketter TA, Post RM. SPECT and PET imaging in mood disorders. J Clin Psychiatry. 1993. 54:6–13.8. Stoleru S, Gregoire M-C, Gerard D, et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav. 1999. 28:1–21.9. Ring HA, George M, Costa DC, Ell PJ. The use of cerebral activation procedures with single photon emission tomography. Eur J Nucl Med. 1991. 18:133–141.10. Stehling MK, Turner R, Mansfield P. Echo-planar imaging; magnetic resonance imaging in a fraction of a second. Science. 1991. 254:43–50.11. Karama S, Lecours AR, Leroux JM, et al. Areas of brain activation in males and females during viewing of erotic film excepts. Human Brain Mapping. 2002. 16:1–13.12. Park K, Seo JJ, Kang HG, Ryu SB, Kim HJ, Jeong GW. A new potential of BOLD fMRI evaluating cerebral centers of penile erection. Int J Impot Res. 2001. 13:73–81.13. Park K, Kang HG, Seo JJ, Kim HJ, Ryu SB, Jeong GW. Blood-Oxygenation-Level-Dependent fMRI for evaluating cerebral regions of female sexual arousal response. Urology. 2001. 57:1189–1194.14. Nemeroff CB, Kilts CD, Berns GS. Functional brain imaging; twenty-first century phrenology or psychobiological advance for the millennium. Am J Psychiatry. 1999. 156:671–673.15. Villringer A, Dirnagl U. Coupling of brain activity and cerebral blood flow; basis of functional neuroimaging. Cerebrovasc Brain Metab Rev. 1995. 7:240–276.16. Redoute J, Stoleru S, Gregoire M-C, Costes N, Cinotti N, Lavenne F, et al. Brain processing of visual sexual stimuli in human males. Human Brain Mapping. 2000. 11:162–177.17. Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow LM. Knobil E, Neill JD, editors. Cellular and molecular mechanisms of female reproductive behaviors. Physiology of reproduction. 1994. New York: Raven Press;107–220.18. Sachs BD, Meisel RL. Knobil E, Neill JD, editors. The physiology of male sexual behavior. Physiology of reproduction. 1994. New York: Raven Press;3–105.19. Allen LS, Hines M, Shryne JE, Gorski RA. Two sexually dimorphic cell groups in the human brain. J Neurosci. 1989. 9:497–506.20. Kupfermann I. Kandel ER, Schwartz JH, Jessell TM, editors. Hypothalamus and limbic system: peptidergic neurons, homeostasis, and emotional behavior. Principles of neural science. 1991. Norwalk: Appleton and Lange;735–749.21. Linas R, Ribary U, Contreras D, Pedroarena C. The neuronal basis for consciousness. Philos Trans R Soc Lond B Biol Sci. 1998. 353:1841–1849.22. McGuire PK, Bench CJ, Frith CD, Marks IM, Frackowiak RSJ, Dolan RJ. Functional anatomy of obsessive-compulsive phenomena. Br J Psychiatry. 1994. 164:459–468.23. Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behavior. Brain. 1995. 118:279–306.24. Beauregard M, Karama S, Leroux JM, Lecours AR, Beaudoin G, Bourgouin P. The functional neuroanatomy of amusement, disgust and sexual arousal. 1998. In : Paper presented at the 4th International Conference on Functional Mapping of the Human Brain; Montreal, Quebec, Canada.25. Lane RD, Reiman EM, Geoffrey LA, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J psychiatry. 1997. 154:926–933.26. Lane RD, Chua PM-L, Dolan RJ. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999. 37:989–997.27. Buchel C, Josephs O, Rees G, Turner R, Frith CD, Friston KJ. The functional anatomy of attention to visual motion: a functional MRI study. Brain. 1998. 121:1281–1294.28. Lane RD, Reiman EM, Bradley MM, et al. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997b. 35:1437–1444.29. O'Craven KM, Rosen BR, Kwong KK, Treisman A, Savoy RL. Voluntary attention modulates fMRI activity in human MTMST. Neuron. 1997. 18:591–598.30. Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996. 382:539–541.31. Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci. 1999. 2:671–676.32. Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color and speed: functional anatomy by positron. 1991.33. Goldenberg G, Podreka I, Steiner M, et al. Contributions of occipital and temporal brain regions to visual and acoustic imagery; a SPECT study. Neuropsychologia. 1991. 29:695–702.34. Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, et al. Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry. 1997. 154:918–925.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroactivation studies using Functional Brain MRI
- An Understanding of Functional MR Imaging
- Functional Magnetic Resonance Imaging with Arterial Spin Labeling: Techniques and Potential Clinical and Research Applications
- Difference of Brain Activation by Visual Erotic Stimuli in Young and Middle-aged Healthy Males
- Functional Magnetic Resonance Imaging of the Brain: Principle and Practical Application