Investig Magn Reson Imaging.
2017 Jun;21(2):91-96. 10.13104/imri.2017.21.2.91.
Functional Magnetic Resonance Imaging with Arterial Spin Labeling: Techniques and Potential Clinical and Research Applications
- Affiliations
-
- 1Department of Radiology, Gyeongsang National University Hospital, Jinju, Korea. choids@gnu.ac.kr
- 2Department of Radiology, Masan University, Changwon, Korea.
- KMID: 2385605
- DOI: http://doi.org/10.13104/imri.2017.21.2.91
Abstract
- PURPOSE
To describe technical methods for functional magnetic resonance imaging (fMRI) study with arterial spin labeling (ASL) compared to blood oxygenation level-dependent (BOLD) technique and discuss the potential of ASL for research and clinical practice.
MATERIALS AND METHODS
Task-based (n = 1) and resting-state fMRI (rs-fMRI) (n = 20) were performed using ASL and BOLD techniques. Results of both techniques were compared.
RESULTS
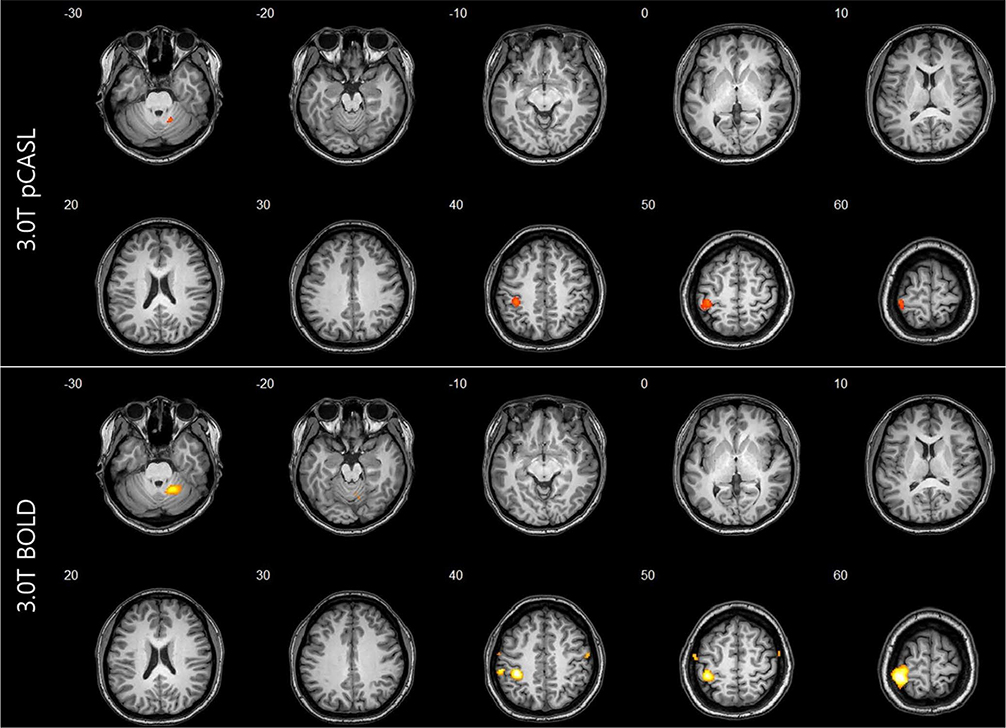
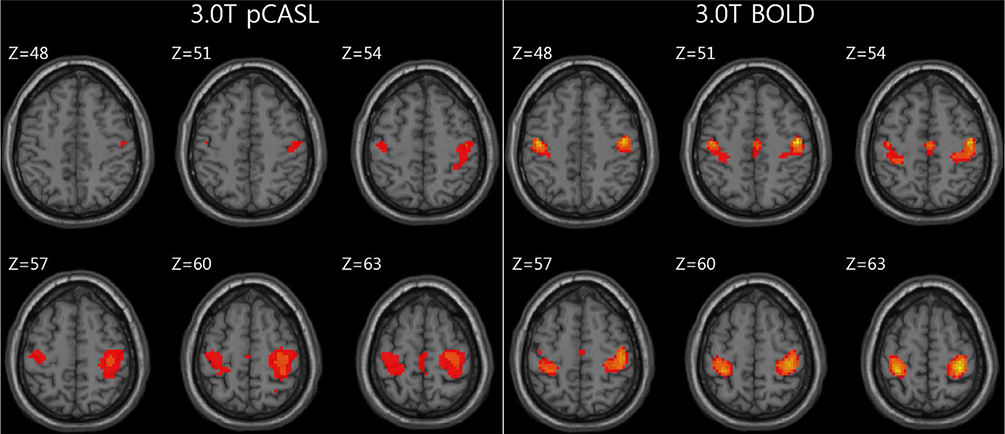
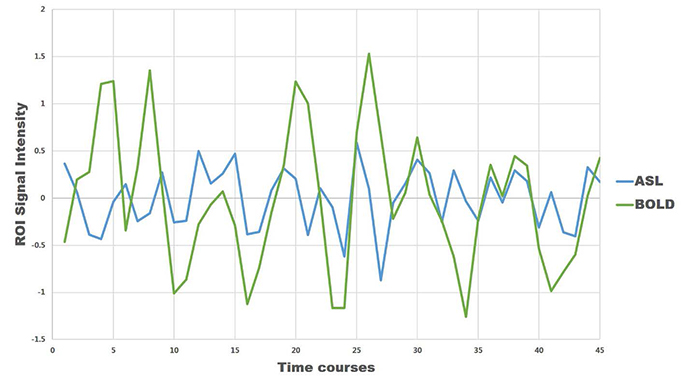
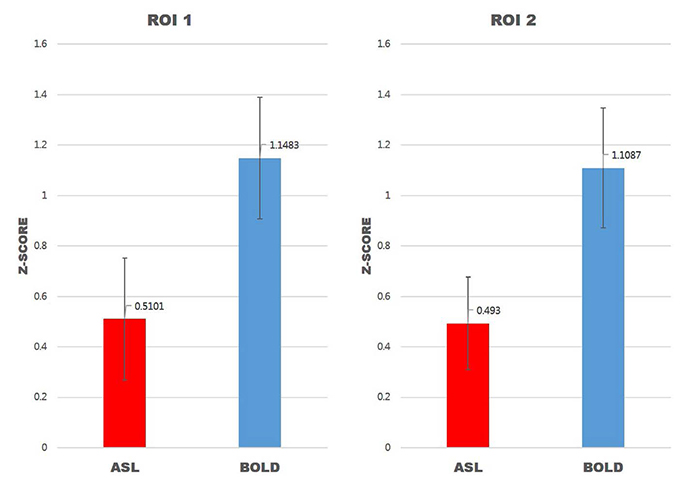
For task-based fMRI with finger-tapping, the primary motor cortex of the contralateral frontal lobe and the ipsilateral cerebellum were activated by both BOLD and ASL fMRI. For rs-fMRI of sensorimotor network, functional connectivity showed similar results between BOLD and ASL.
CONCLUSION
ASL technique has potential application in clinical and research fields because all brain perfusion imaging, CBF measurement, and rs-fMRI study can be performed in a single acquisition.
Keyword
MeSH Terms
Figure
Reference
-
1. Pollock JM, Tan H, Kraft RA, Whitlow CT, Burdette JH, Maldjian JA. Arterial spin-labeled MR perfusion imaging: clinical applications. Magn Reson Imaging Clin N Am. 2009; 17:315–338.2. Jahng GH, Li KL, Ostergaard L, Calamante F. Perfusion magnetic resonance imaging: a comprehensive update on principles and techniques. Korean J Radiol. 2014; 15:554–577.3. Kim SM, Kim MJ, Rhee HY, et al. Regional cerebral perfusion in patients with Alzheimer's disease and mild cognitive impairment: effect of APOE epsilon4 allele. Neuroradiology. 2013; 55:25–34.4. Choi YJ, Kim HS, Jahng GH, Kim SJ, Suh DC. Pseudoprogression in patients with glioblastoma: added value of arterial spin labeling to dynamic susceptibility contrast perfusion MR imaging. Acta Radiol. 2013; 54:448–454.5. Zhu S, Fang Z, Hu S, Wang Z, Rao H. Resting state brain function analysis using concurrent BOLD in ASL perfusion fMRI. PLoS One. 2013; 8:e65884.6. Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB toolbox for “Pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010; 4:13.7. Wang J, Aguirre GK, Kimberg DY, Roc AC, Li L, Detre JA. Arterial spin labeling perfusion fMRI with very low task frequency. Magn Reson Med. 2003; 49:796–802.8. Kallioniemi E, Pitkanen M, Kononen M, Vanninen R, Julkunen P. Localization of cortical primary motor area of the hand using navigated transcranial magnetic stimulation, BOLD and arterial spin labeling fMRI. J Neurosci Methods. 2016; 273:138–148.9. Fleisher AS, Podraza KM, Bangen KJ, et al. Cerebral perfusion and oxygenation differences in Alzheimer's disease risk. Neurobiol Aging. 2009; 30:1737–1748.10. Chen JJ, Jann K, Wang DJ. Characterizing resting-state brain function using arterial spin labeling. Brain Connect. 2015; 5:527–542.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Arterial Spin Labelling-Based Blood-Brain Barrier Assessment and Its Applications
- Pseudo Continuous Arterial Spin Labeling MR Imaging of Status Epilepticus
- Perfusion Magnetic Resonance Imaging: A Comprehensive Update on Principles and Techniques
- Magnetic Resonance Imaging Meets Fiber Optics: a Brief Investigation of Multimodal Studies on Fiber OpticsBased Diagnostic / Therapeutic Techniques and Magnetic Resonance Imaging
- Arterial Spin Labelling Perfusion, Proton MR Spectroscopy and Susceptibility-Weighted MR Findings of Acute Necrotizing Encephalopathy: a Case Report