J Korean Med Sci.
2024 Mar;39(8):e72. 10.3346/jkms.2024.39.e72.
Comparison of High- and Low-Dose Rivaroxaban Regimens in Elderly East Asian Patients With Atrial Fibrillation
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Heart Vascular and Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Division of Cardiology, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Division of Cardiology, Department of Internal Medicine, Korea University College of Medicine and Korea University Anam Hospital, Seoul, Korea
- 4Department of Internal Medicine, Seoul National University College of Medicine and Seoul National University Hospital, Seoul, Korea
- 5Inha University College of Medicine and Inha University Hospital, Incheon, Korea
- 6Cardiovascular Center, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- KMID: 2553325
- DOI: http://doi.org/10.3346/jkms.2024.39.e72
Abstract
- Background
In the Rivaroxaban Once-daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) trial, rivaroxaban 20 mg was the on-label dose, and the dose-reduction criterion for rivaroxaban was a creatinine clearance of < 50 mL/min. Some Asian countries are using reduced doses label according to the J-ROCKET AF trial. The aim of this study was to assess the safety and efficacy of a high-dose rivaroxaban regimen (HDRR, 20/15 mg) and low-dose rivaroxaban regimen (LDRR, 15/10 mg) among elderly East Asian patients with atrial fibrillation (AF) in real-world practice.
Methods
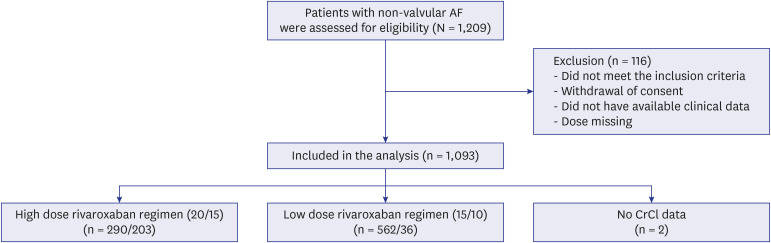
This study was a multicenter, prospective, non-interventional observational study designed to evaluate the efficacy and safety of rivaroxaban in AF patients > 65 years of age with or without renal impairment.
Results
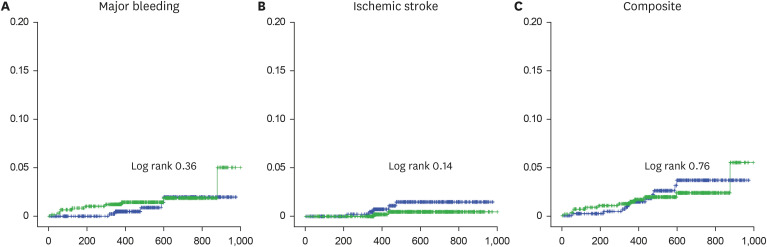
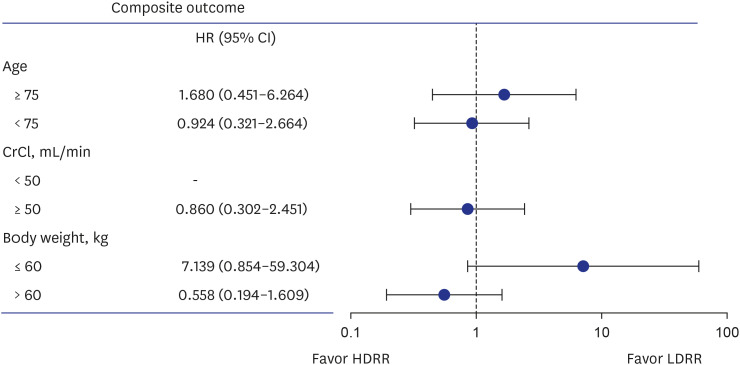
A total of 1,093 patients (mean age, 72.8 ± 5.8 years; 686 [62.9%] men) were included in the analysis, with 493 patients allocated to the HDRR group and 598 patients allocated to the LDRR group. A total of 765 patients received 15 mg of rivaroxaban (203 in the HDRR group and 562 in the LDRR group). There were no significant differences in the incidence rates of major bleeding (adjusted hazard ratio [HR], 0.64; 95% confidential interval [CI], 0.21–1.93), stroke (adjusted HR, 3.21; 95% CI, 0.54–19.03), and composite outcomes (adjusted HR, 1.13; 95% CI, 0.47–2.69) between the HDRR and LDRR groups.
Conclusion
This study revealed the safety and effectiveness of either dose regimen of rivaroxaban in an Asian population for stroke prevention of AF. Considerable numbers of patients are receiving LDRR therapy in real-world practice in Asia. Both regimens were safe and effective for these patients.
Keyword
Figure
Reference
-
1. Lee SR, Choi EK, Han KD, Cha MJ, Oh S. Trends in the incidence and prevalence of atrial fibrillation and estimated thromboembolic risk using the CHA2DS2-VASc score in the entire Korean population. Int J Cardiol. 2017; 236:226–231. PMID: 28233629.2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med. 1987; 147(9):1561–1564. PMID: 3632164.3. Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007; 115(21):2689–2696. PMID: 17515465.4. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014; 383(9921):955–962. PMID: 24315724.5. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365(10):883–891. PMID: 21830957.6. Halperin JL, Hankey GJ, Wojdyla DM, Piccini JP, Lokhnygina Y, Patel MR, et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation. 2014; 130(2):138–146. PMID: 24895454.7. Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation – the J-ROCKET AF study –. Circ J. 2012; 76(9):2104–2111. PMID: 22664783.8. Schulman S, Angerås U, Bergqvist D, Eriksson B, Lassen MR, Fisher W, et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010; 8(1):202–204. PMID: 19878532.9. Lee SR, Lee YS, Park JS, Cha MJ, Kim TH, Park J, et al. Label adherence for non-vitamin K Antagonist oral anticoagulants in a prospective cohort of Asian patients with atrial fibrillation. Yonsei Med J. 2019; 60(3):277–284. PMID: 30799590.10. Chan YH, Lee HF, Wang CL, Chang SH, Yeh CH, Chao TF, et al. Comparisons of rivaroxaban following different dosage criteria (ROCKET AF or J-ROCKET AF Trials) in Asian patients with atrial fibrillation. J Am Heart Assoc. 2019; 8(21):e013053. PMID: 31623498.11. Lee SR, Choi EK, Han KD, Jung JH, Oh S, Lip GY. Optimal rivaroxaban dose in Asian patients with atrial fibrillation and normal or mildly impaired renal function. Stroke. 2019; 50(5):1140–1148. PMID: 30913984.12. Liu XQ, Li ZR, Wang CY, Chen YT, Jiao Z. Is a lower dose of rivaroxaban required for Asians? A systematic review of a population pharmacokinetics and pharmacodynamics analysis of rivaroxaban. Pharmaceutics. 2023; 15(2):588. PMID: 36839909.13. Ikeda T, Ogawa S, Kitazono T, Nakagawara J, Minematsu K, Miyamoto S, et al. Real-world outcomes of the Xarelto Post-Authorization Safety & Effectiveness Study in Japanese Patients with Atrial Fibrillation (XAPASS). J Cardiol. 2019; 74(1):60–66. PMID: 30745002.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of New Oral Anticoagulants: Prevention of Stroke in Patients with Nonvalvular Atrial Fibrillation
- Real World Comparison of Rivaroxaban and Warfarin in Korean Patients with Atrial Fibrillation: Propensity Matching Cohort Analysis
- Non-Vitamin K Antagonist Oral Anticoagulants in Medical Conditions at High Risk of Thromboembolism beyond Atrial Fibrillation
- Hemorrhagic pericarditis associated with rivaroxaban in an atrial fibrillation patient with pacemaker
- Long-Term Anticoagulation in the Extreme Elderly with the Newer Antithrombotics: Safe or Sorry?