Nutr Res Pract.
2024 Feb;18(1):46-61. 10.4162/nrp.2024.18.1.46.
Animal protein hydrolysate reduces visceral fat and inhibits insulin resistance and hepatic steatosis in aged mice
- Affiliations
-
- 1Department of Food Science and Nutrition, Kyungpook National University, Daegu 41566, Korea
- 2Center for Food and Nutritional Genomics Research, Kyungpook National University, Daegu 41566, Korea
- 3Bio Convergence Testing Center, Daegu Haany University, Gyeongsan 38610, Korea
- 4Center for Beautiful Aging, Kyungpook National University, Daegu 41566, Korea
- KMID: 2552278
- DOI: http://doi.org/10.4162/nrp.2024.18.1.46
Abstract
- BACKGROUND/OBJECTIVES
An increasing life expectancy in society has burdened healthcare systems substantially because of the rising prevalence of age-related metabolic diseases. This study compared the effects of animal protein hydrolysate (APH) and casein on metabolic diseases using aged mice.
MATERIALS/METHODS
Eight-week-old and 50-week-old C57BL/6J mice were used as the non-aged (YC group) and aged controls (NC group), respectively. The aged mice were divided randomly into 3 groups (NC, low-APH [LP], and high-APH [HP] and fed each experimental diet for 12 weeks. In the LP and HP groups, casein in the AIN-93G diet was substituted with 16 kcal% and 24 kcal% APH, respectively. The mice were sacrificed when they were 63-weekold, and plasma and hepatic lipid, white adipose tissue weight, hepatic glucose, lipid, and antioxidant enzyme activities, immunohistochemistry staining, and mRNA expression related to the glucose metabolism on liver and muscle were analyzed.
RESULTS
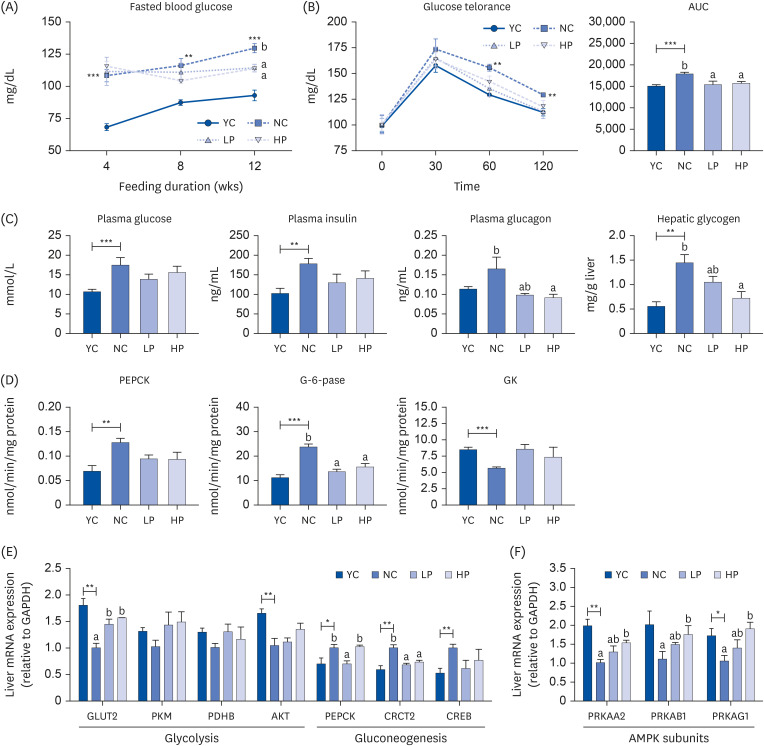
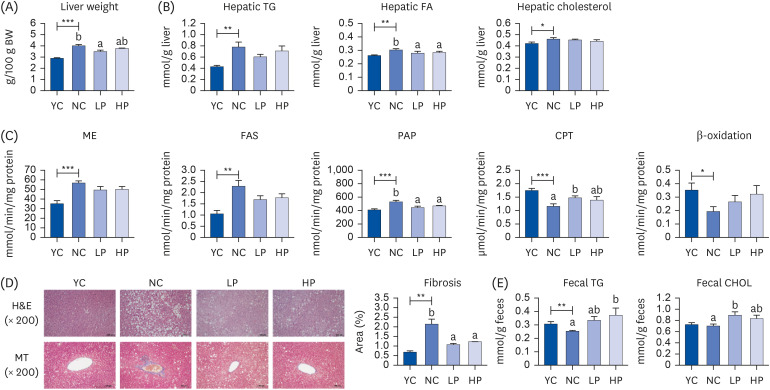
Supplementation of APH in aging mice resulted in a significant decrease in visceral fat (epididymal, perirenal, retroperitoneal, and mesenteric fat) compared to the negative control (NC) group. The intraperitoneal glucose tolerance test and area under the curve analysis revealed insulin resistance in the NC group, which was alleviated by APH supplementation. APH supplementation reduced hepatic gluconeogenesis and increased glucose utilization in the liver and muscle. Furthermore, APH supplementation improved hepatic steatosis by reducing the hepatic fatty acid and phosphatidate phosphatase activity while increasing the hepatic carnitine palmitoyltransferase activity. Furthermore, in the APH supplementation groups, the red blood cell (RBC) thiobarbituric acid reactive substances and hepatic H 2 O 2 levels decreased, and the RBC glutathione, hepatic catalase, and glutathione peroxidase activities increased.
CONCLUSIONS
APH supplementation reduced visceral fat accumulation and alleviated obesity-related metabolic diseases, including insulin resistance and hepatic steatosis, in aged mice. Therefore, high-quality animal protein APH that reduces the molecular weight and enhances the protein digestibility-corrected amino acid score has potential as a dietary supplement for healthy aging.
Keyword
Figure
Reference
-
1. Guo J, Huang X, Dou L, Yan M, Shen T, Tang W, Li J. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct Target Ther. 2022; 7:391. PMID: 36522308.2. Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009; 8:339–348. PMID: 19576300.3. Jura M, Kozak LP. Obesity and related consequences to ageing. Age (Dordr). 2016; 38:23. PMID: 26846415.4. Folsom AR, Kaye SA, Sellers TA, Hong CP, Cerhan JR, Potter JD, Prineas RJ. Body fat distribution and 5-year risk of death in older women. JAMA. 1993; 269:483–487. PMID: 8419667.5. Brochu M, Tchernof A, Dionne IJ, Sites CK, Eltabbakh GH, Sims EA, Poehlman ET. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001; 86:1020–1025. PMID: 11238480.6. Gan L, Chitturi S, Farrell GC. Mechanisms and implications of age-related changes in the liver: nonalcoholic Fatty liver disease in the elderly. Curr Gerontol Geriatr Res. 2011; 2011:831536. PMID: 21918648.7. McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992; 258:766–770. PMID: 1439783.8. Lytrivi M, Castell AL, Poitout V, Cnop M. Recent insights into mechanisms of β-cell lipo- and glucolipotoxicity in type 2 diabetes. J Mol Biol. 2020; 432:1514–1534. PMID: 31628942.9. Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic β-cell death. Trends Endocrinol Metab. 2011; 22:266–274. PMID: 21458293.10. Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009; 12:86–90. PMID: 19057193.11. Bartali B, Frongillo EA, Stipanuk MH, Bandinelli S, Salvini S, Palli D, Morais JA, Volpato S, Guralnik JM, Ferrucci L. Protein intake and muscle strength in older persons: does inflammation matter? J Am Geriatr Soc. 2012; 60:480–484. PMID: 22283208.12. Hackney KJ, Trautman K, Johnson N, Mcgrath R, Stastny S. Protein and muscle health during aging: benefits and concerns related to animal-based protein. Anim Front. 2019; 9:12–17.13. Lim MT, Pan BJ, Toh DW, Sutanto CN, Kim JE. Animal protein versus plant protein in supporting lean mass and muscle strength: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2021; 13:661. PMID: 33670701.14. Schaafsma G. Advantages and limitations of the protein digestibility-corrected amino acid score (PDCAAS) as a method for evaluating protein quality in human diets. Br J Nutr. 2012; 108(Suppl 2):S333–S336. PMID: 23107546.15. Carroll NV, Longley RW, Roe JH. The determination of glycogen in liver and muscle by use of anthrone reagent. J Biol Chem. 1956; 220:583–593. PMID: 13331917.16. Bentle LA, Lardy HA. Interaction of anions and divalent metal ions with phosphoenolpyruvate carboxykinase. J Biol Chem. 1976; 251:2916–2921. PMID: 1270433.17. Alegre M, Ciudad CJ, Fillat C, Guinovart JJ. Determination of glucose-6-phosphatase activity using the glucose dehydrogenase-coupled reaction. Anal Biochem. 1988; 173:185–189. PMID: 2847588.18. Davidson AL, Arion WJ. Factors underlying significant underestimations of glucokinase activity in crude liver extracts: physiological implications of higher cellular activity. Arch Biochem Biophys. 1987; 253:156–167. PMID: 3813560.19. Nepokroeff CM, Lakshmanan MR, Porter JW. Fatty-acid synthase from rat liver. Methods Enzymol. 1975; 35:37–44. PMID: 1121291.20. Markwell MA, McGroarty EJ, Bieber LL, Tolbert NE. The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. A new peroxisomal enzyme. J Biol Chem. 1973; 248:3426–3432. PMID: 4702872.21. Lazarow PB. Assay of peroxisomal beta-oxidation of fatty acids. Methods Enzymol. 1981; 72:315–319. PMID: 7031421.22. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358. PMID: 36810.23. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974; 47:469–474. PMID: 4215654.24. Aebi H. Catalase in vitro . Methods Enzymol. 1984; 105:121–126. PMID: 6727660.25. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70:158–169. PMID: 6066618.26. Pinto RE, Bartley W. The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem J. 1969; 112:109–115. PMID: 4388243.27. Graham A, Hassall DG, Rafique S, Owen JS. Evidence for a paraoxonase-independent inhibition of low-density lipoprotein oxidation by high-density lipoprotein. Atherosclerosis. 1997; 135:193–204. PMID: 9430369.28. Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, Scherer P, Rossetti L, Barzilai N. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002; 51:2951–2958. PMID: 12351432.29. Kim YW, Kim JY, Lee SK. Surgical removal of visceral fat decreases plasma free fatty acid and increases insulin sensitivity on liver and peripheral tissue in monosodium glutamate (MSG)-obese rats. J Korean Med Sci. 1999; 14:539–545. PMID: 10576150.30. Jiang Y, Zhang Y, Jin M, Gu Z, Pei Y, Meng P. Aged-related changes in body composition and association between body composition with bone mass density by body mass index in chinese han men over 50-year-old. PLoS One. 2015; 10:e0130400. PMID: 26090818.31. Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004; 286:E92–101. PMID: 14506079.32. Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. Biochim Biophys Acta. 2009; 1790:1117–1123. PMID: 19364483.33. Tan CY, Vidal-Puig A. Adipose tissue expandability: the metabolic problems of obesity may arise from the inability to become more obese. Biochem Soc Trans. 2008; 36:935–940. PMID: 18793164.34. Slawik M, Vidal-Puig AJ. Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res Rev. 2006; 5:144–164. PMID: 16630750.35. Rudman D, Feller AG, Cohn L, Shetty KR, Rudman IW, Draper MW. Effects of human growth hormone on body composition in elderly men. Horm Res. 1991; 36(Suppl 1):73–81. PMID: 1806490.36. Navale AM, Paranjape AN. Glucose transporters: physiological and pathological roles. Biophys Rev. 2016; 8:5–9.37. Han HS, Kang G, Kim JS, Choi BH, Koo SH. Regulation of glucose metabolism from a liver-centric perspective. Exp Mol Med. 2016; 48:e218. PMID: 26964834.38. Li X, Sui Y, Wu Q, Xie B, Sun Z. Attenuated mTOR signaling and enhanced glucose homeostasis by dietary supplementation with lotus seedpod oligomeric procyanidins in streptozotocin (STZ)-induced diabetic mice. J Agric Food Chem. 2017; 65:3801–3810. PMID: 28314100.39. Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev. 2013; 23:53–62. PMID: 23317514.40. Chen Z, Yu Y, Cai J, Li H. Emerging molecular targets for treatment of nonalcoholic fatty liver disease. Trends Endocrinol Metab. 2019; 30:903–914. PMID: 31597607.41. Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. 2020; 152:116–141. PMID: 32156524.42. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018; 24:908–922. PMID: 29967350.43. Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, Herder C, Carstensen M, Krausch M, Knoefel WT, et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015; 21:739–746. PMID: 25955209.44. Videla LA, Rodrigo R, Orellana M, Fernandez V, Tapia G, Quiñones L, Varela N, Contreras J, Lazarte R, Csendes A, et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci (Lond). 2004; 106:261–268. PMID: 14556645.45. Köroğlu E, Canbakan B, Atay K, Hatemi İ, Tuncer M, Dobrucalı A, Sonsuz A, Gültepe I, Şentürk H. Role of oxidative stress and insulin resistance in disease severity of non-alcoholic fatty liver disease. Turk J Gastroenterol. 2016; 27:361–366. PMID: 27458852.46. Masarone M, Rosato V, Dallio M, Gravina AG, Aglitti A, Loguercio C, Federico A, Persico M. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2018; 2018:9547613. PMID: 29991976.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of the Combination of Evogliptin and Leucine on Insulin Resistance and Hepatic Steatosis in High-Fat Diet-Fed Mice
- The SCAP/SREBP Pathway: A Mediator of Hepatic Steatosis
- Umbelliferone Ameliorates Hepatic Steatosis and Lipid-Induced ER Stress in High-Fat Diet-Induced Obese Mice
- Characteristics of the Newly Established Diabetic Model Mice, TallyHo
- Myeloid-specific SIRT1 Deletion Aggravates Hepatic Inflammation and Steatosis in High-fat Diet-fed Mice