Korean J Physiol Pharmacol.
2015 Sep;19(5):451-460. 10.4196/kjpp.2015.19.5.451.
Myeloid-specific SIRT1 Deletion Aggravates Hepatic Inflammation and Steatosis in High-fat Diet-fed Mice
- Affiliations
-
- 1Department of Anatomy and Convergence Medical Science, Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju 660-751, Korea. anaroh@gnu.ac.kr
- KMID: 2285590
- DOI: http://doi.org/10.4196/kjpp.2015.19.5.451
Abstract
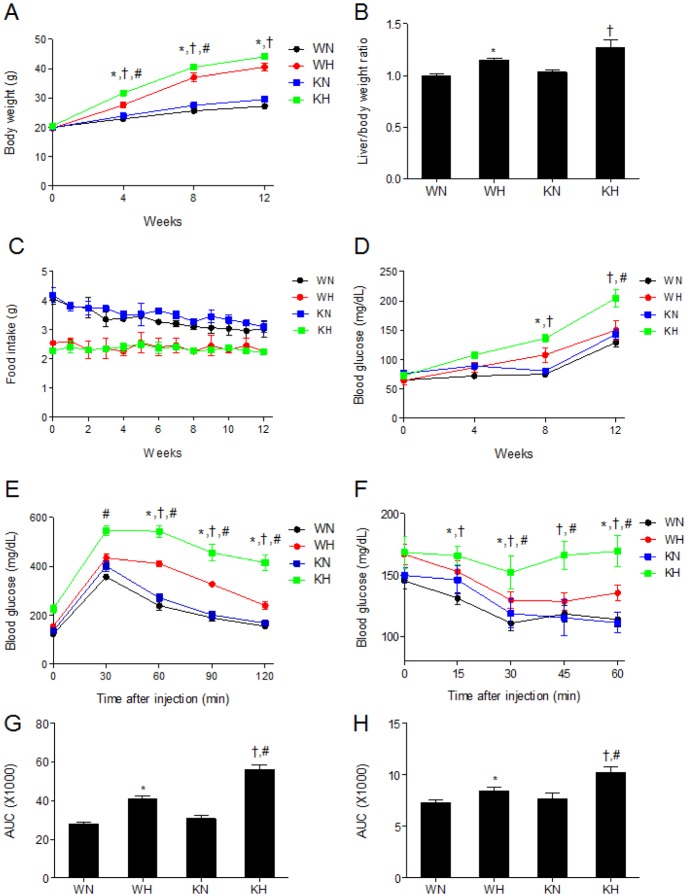
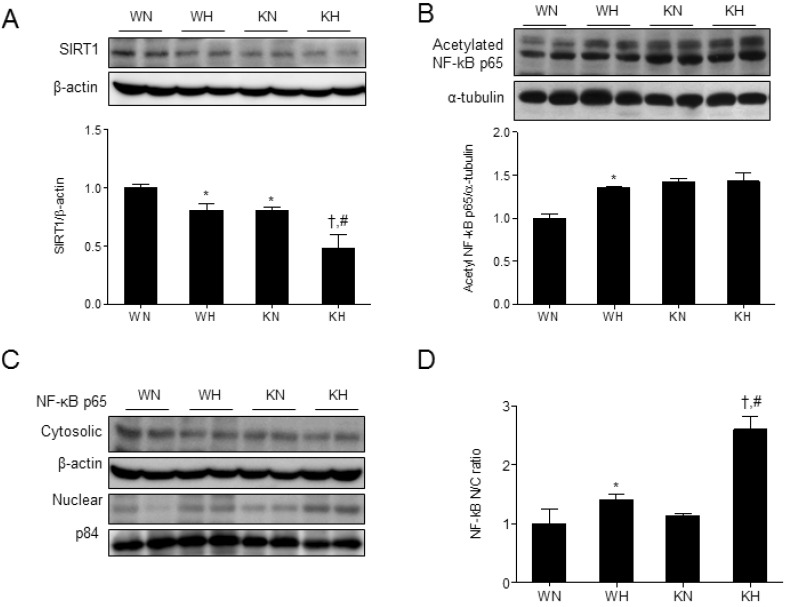
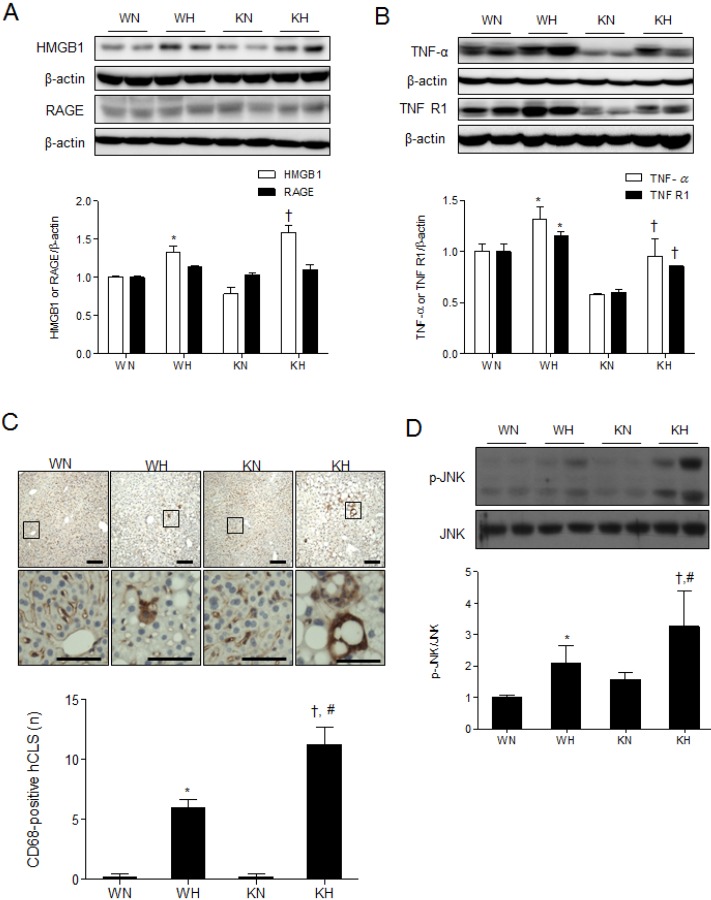
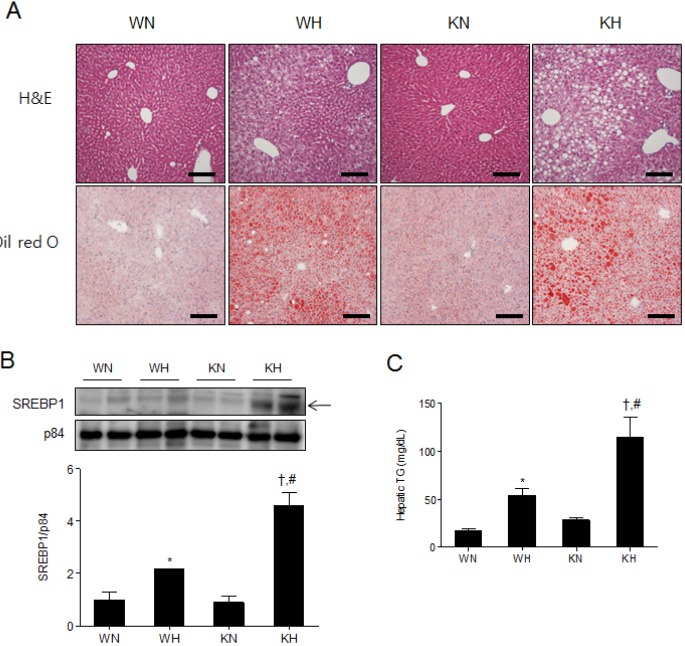
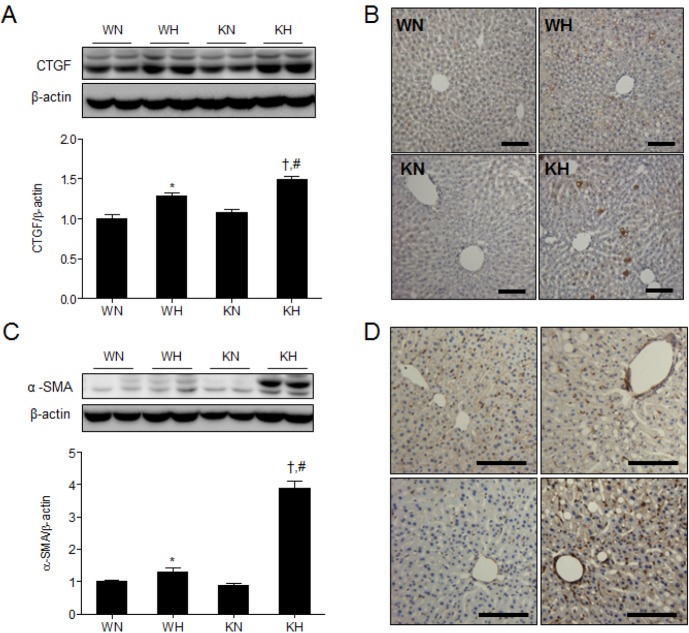
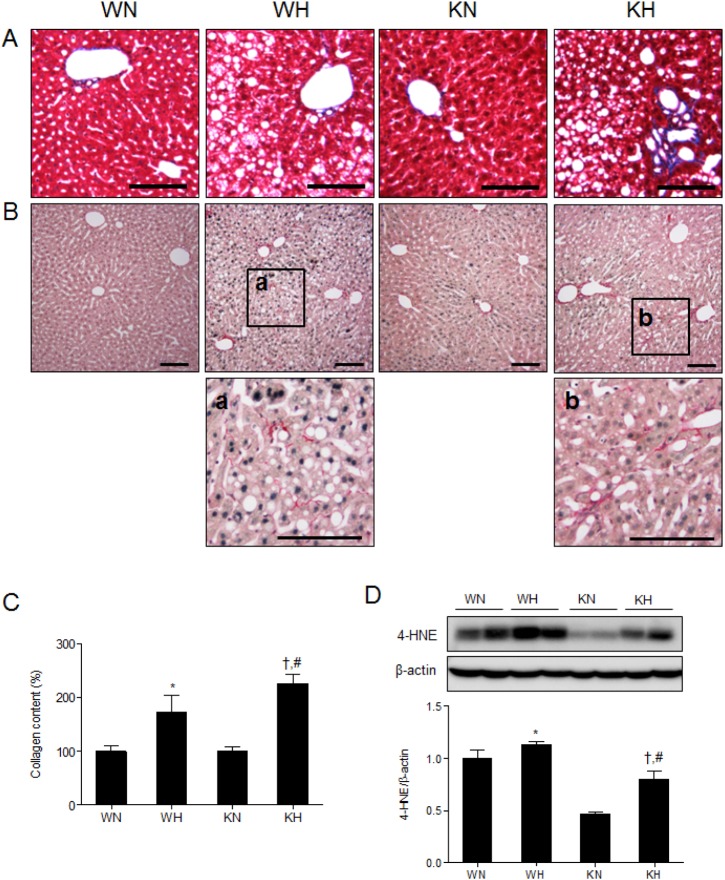
- Sirtuin 1 (SIRT1) is a mammalian NAD+-dependent protein deacetylase that regulates cellular metabolism and inflammatory response. The organ-specific deletion of SIRT1 induces local inflammation and insulin resistance in dietary and genetic obesity. Macrophage-mediated inflammation contributes to insulin resistance and metabolic syndrome, however, the macrophage-specific SIRT1 function in the context of obesity is largely unknown. C57/BL6 wild type (WT) or myeloid-specific SIRT1 knockout (KO) mice were fed a high-fat diet (HFD) or normal diet (ND) for 12 weeks. Metabolic parameters and markers of hepatic steatosis and inflammation in liver were compared in WT and KO mice. SIRT1 deletion enhanced HFD-induced changes on body and liver weight gain, and increased glucose and insulin resistance. In liver, SIRT1 deletion increased the acetylation, and enhanced HFD-induced nuclear translocation of nuclear factor kappa B (NF-kappaB), hepatic inflammation and macrophage infiltration. HFD-fed KO mice showed severe hepatic steatosis by activating lipogenic pathway through sterol regulatory element-binding protein 1 (SREBP-1), and hepatic fibrogenesis, as indicated by induction of connective tissue growth factor (CTGF), alpha-smooth muscle actin (alpha-SMA), and collagen secretion. Myeloid-specific deletion of SIRT1 stimulates obesity-induced inflammation and increases the risk of hepatic fibrosis. Targeted induction of macrophage SIRT1 may be a good therapy for alleviating inflammation-associated metabolic syndrome.
MeSH Terms
-
Acetylation
Actins
Animals
Collagen
Connective Tissue Growth Factor
Diet
Diet, High-Fat
Fibrosis
Glucose
Inflammation*
Insulin Resistance
Liver
Macrophages
Metabolism
Mice*
NF-kappa B
Obesity
Sirtuin 1
Sterol Regulatory Element Binding Protein 1
Weight Gain
Actins
Collagen
Connective Tissue Growth Factor
Glucose
NF-kappa B
Sirtuin 1
Sterol Regulatory Element Binding Protein 1
Figure
Reference
-
1. Li X. SIRT1 and energy metabolism. Acta Biochim Biophys Sin (Shanghai). 2013; 45:51–60. PMID: 23257294.
Article2. Smith JS, Brachmann CB, Celic I, Kenna MA, Muhammad S, Starai VJ, Avalos JL, Escalante-Semerena JC, Grubmeyer C, Wolberger C, Boeke JD. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc Natl Acad Sci U S A. 2000; 97:6658–6663. PMID: 10841563.
Article3. Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012; 13:225–238. PMID: 22395773.
Article4. Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, Purushotham A, Li X. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol. 2010; 30:4712–4721. PMID: 20647536.
Article5. Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004; 23:2369–2380. PMID: 15152190.6. Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004; 305:390–392. PMID: 15205477.
Article7. Anastasiou D, Krek W. SIRT1: linking adaptive cellular responses to aging-associated changes in organismal physiology. Physiology (Bethesda). 2006; 21:404–410. PMID: 17119153.
Article8. Cohen DE, Supinski AM, Bonkowski MS, Donmez G, Guarente LP. Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev. 2009; 23:2812–2817. PMID: 20008932.
Article9. Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009; 9:327–338. PMID: 19356714.
Article10. Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A. 2007; 104:12861–12866. PMID: 17646659.
Article11. Chawla A. Control of macrophage activation and function by PPARs. Circ Res. 2010; 106:1559–1569. PMID: 20508200.
Article12. Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006; 281:26602–26614. PMID: 16809344.
Article13. Deng XQ, Chen LL, Li NX. The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int. 2007; 27:708–715. PMID: 17498258.
Article14. Yoshizaki T, Schenk S, Imamura T, Babendure JL, Sonoda N, Bae EJ, Oh DY, Lu M, Milne JC, Westphal C, Bandyopadhyay G, Olefsky JM. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am J Physiol Endocrinol Metab. 2010; 298:E419–E428. PMID: 19996381.
Article15. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010; 72:219–246. PMID: 20148674.
Article16. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112:1796–1808. PMID: 14679176.
Article17. Barnes PJ, Adcock IM, Ito K. Histone acetylation and deacetylation: importance in inflammatory lung diseases. Eur Respir J. 2005; 25:552–563. PMID: 15738302.
Article18. Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y, Han JY, Kato S, Shimoda M, Oike Y, Tomizawa M, Makino S, Ohkura T, Saito H, Kumagai N, Nagata H, Ishii H, Hibi T. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006; 55:415–424. PMID: 16174657.19. Itoh M, Kato H, Suganami T, Konuma K, Marumoto Y, Terai S, Sakugawa H, Kanai S, Hamaguchi M, Fukaishi T, Aoe S, Akiyoshi K, Komohara Y, Takeya M9, Sakaida I, Ogawa Y. Hepatic crown-like structure: a unique histological feature in non-alcoholic steatohepatitis in mice and humans. PLoS One. 2013; 8:e82163. PMID: 24349208.
Article20. Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013; 339:218–222. PMID: 23223452.
Article21. den Boer M, Voshol PJ, Kuipers F, Havekes LM, Romijn JA. Hepatic steatosis: a mediator of the metabolic syndrome. Lessons from animal models. Arterioscler Thromb Vasc Biol. 2004; 24:644–649. PMID: 14715643.
Article22. Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, Conti M, Huet S, Ba N, Buffet C, Bedossa P. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001; 34:738–744. PMID: 11584370.
Article23. Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007; 117:539–548. PMID: 17332881.
Article24. Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013; 7:e330–e341. PMID: 24455761.
Article25. Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest. 2011; 121:4477–4490. PMID: 21965330.
Article26. Svegliati Baroni G, D'Ambrosio L, Ferretti G, Casini A, Di Sario A, Salzano R, Ridolfi F, Saccomanno S, Jezequel AM, Benedetti A. Fibrogenic effect of oxidative stress on rat hepatic stellate cells. Hepatology. 1998; 27:720–726. PMID: 9500700.
Article27. Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008; 177:861–870. PMID: 18174544.
Article28. Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009; 29:1363–1374. PMID: 19103747.
Article29. Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008; 105:9793–9798. PMID: 18599449.
Article30. Hah YS, Cheon YH, Lim HS, Cho HY, Park BH, Ka SO, Lee YR, Jeong DW, Kim HO, Han MK, Lee SI. Myeloid deletion of SIRT1 aggravates serum transfer arthritis in mice via nuclear factor-κB activation. PLoS One. 2014; 9:e87733. PMID: 24498364.
Article31. Purushotham A, Xu Q, Li X. Systemic SIRT1 insufficiency results in disruption of energy homeostasis and steroid hormone metabolism upon high-fat-diet feeding. FASEB J. 2012; 26:656–667. PMID: 22006157.
Article32. Xu F, Gao Z, Zhang J, Rivera CA, Yin J, Weng J, Ye J. Lack of SIRT1 (Mammalian Sirtuin 1) activity leads to liver steatosis in the SIRT1+/- mice: a role of lipid mobilization and inflammation. Endocrinology. 2010; 151:2504–2514. PMID: 20339025.
Article33. Williams EJ, Gaça MD, Brigstock DR, Arthur MJ, Benyon RC. Increased expression of connective tissue growth factor in fibrotic human liver and in activated hepatic stellate cells. J Hepatol. 2000; 32:754–761. PMID: 10845662.
Article34. Kim S, Jung J, Kim H, Heo RW, Yi CO, Lee JE, Jeon BT, Kim WH, Hahm JR, Roh GS. Exendin-4 improves nonalcoholic fatty liver disease by regulating glucose transporter 4 expression in ob/ob mice. Korean J Physiol Pharmacol. 2014; 18:333–339. PMID: 25177166.
Article35. Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996; 271:665–668. PMID: 8571133.36. Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001; 280:E745–E751. PMID: 11287357.
Article37. De Taeye BM, Novitskaya T, McGuinness OP, Gleaves L, Medda M, Covington JW, Vaughan DE. Macrophage TNF-alpha contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am J Physiol Endocrinol Metab. 2007; 293:E713–E725. PMID: 17578885.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of high-fat diet on the testicular function of sirtuin 1 (SIRT1) knockout male mice
- Effects of the Combination of Evogliptin and Leucine on Insulin Resistance and Hepatic Steatosis in High-Fat Diet-Fed Mice
- Umbelliferone Ameliorates Hepatic Steatosis and Lipid-Induced ER Stress in High-Fat Diet-Induced Obese Mice
- Mentha canadensis attenuates adiposity and hepatic steatosis in high-fat diet-induced obese mice
- Efficacy of evogliptin and cenicriviroc against nonalcoholic steatohepatitis in mice: a comparative study