Nutr Res Pract.
2024 Feb;18(1):19-32. 10.4162/nrp.2024.18.1.19.
Effects of an in vitro vitamin D treatment on the inflammatory responses in visceral adipose tissue from Ldlr −/− mice
- Affiliations
-
- 1Department of Food and Nutrition, College of Human Ecology, Seoul National University, Seoul 08826, Korea
- 2Research Institute of Human Ecology, College of Human Ecology, Seoul National University, Seoul 08826, Korea
- KMID: 2552271
- DOI: http://doi.org/10.4162/nrp.2024.18.1.19
Abstract
- BACKGROUND/OBJECTIVES
Atherosclerosis is associated with increased inflammation in the visceral adipose tissue (VAT). Vitamin D has been reported to modulate the inflammatory responses of stromal vascular cells (SVCs) and adipocytes in adipose tissue, but the role of vitamin D in atherosclerosis biology is unclear. This study examined the effects of in vitro 1,25-dihydroxyvitamin D 3 (1,25[OH] 2 D 3 ) treatment on the inflammatory responses of SVCs and adipocytes from atherosclerotic mice.
MATERIALS/METHODS
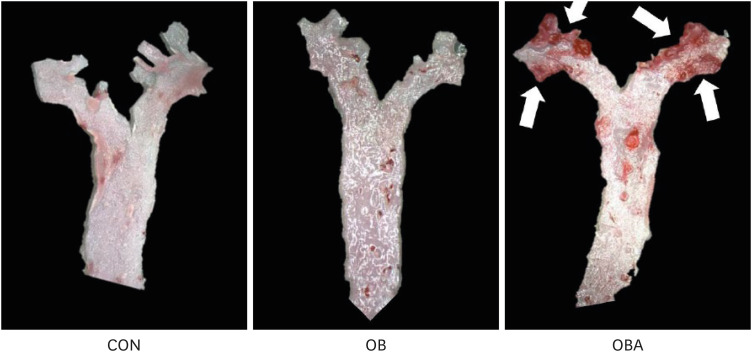
C57BL/6J (B6) mice were divided randomly into 2 groups and fed a 10% kcal fat control diet (control group, CON) or 41% kcal fat, 0.21% cholesterol (high fat+ cholesterol, HFC) diet (obese group, OB), and B6.129S7-Ldlr tm1Her /J (Ldlr −/− ) mice were fed a HFC diet (obese with atherosclerosis group, OBA) for 16 weeks. SVCs and adipocytes isolated from VAT were pre-incubated with 1,25(OH) 2 D 3 for 24 h and stimulated with lipopolysaccarides for the next 24 h. Proinflammatory cytokine production by adipocytes and SVCs, the immune cell population in SVCs, and the expression of the genes involved in the inflammatory signaling pathway in SVCs were determined.
RESULTS
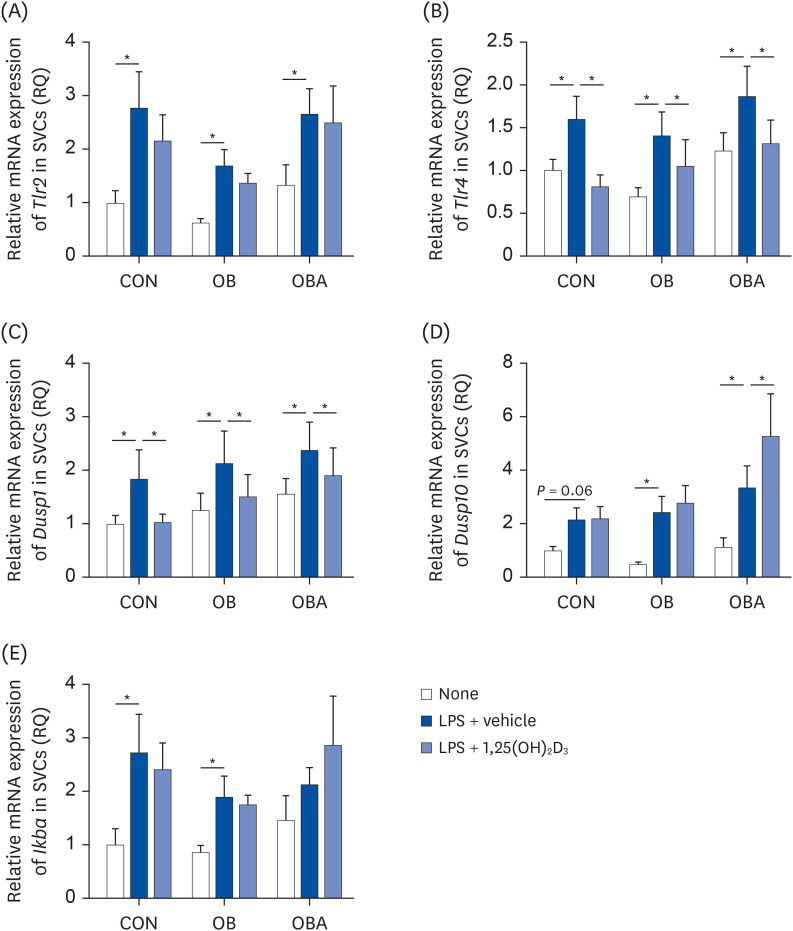
The numbers of total macrophages and SVCs per mouse were higher in OB and OBA groups than the CON group. The in vitro 1,25(OH) 2 D 3 treatment significantly reduced macrophages/SVCs (%) in the OBA group. Consistent with this change, the production of interleukin-6 and monocyte chemoattractant protein 1 (MCP-1) by SVCs from the OBA group was decreased by 1,25(OH) 2 D 3 treatment. The 1,25(OH) 2 D 3 treatment significantly reduced the toll-like receptor 4 and dual-specificity protein phosphatase 1 (also known as mitogenactivated protein kinase phosphatase 1) mRNA levels in SVCs and MCP-1 production by adipocytes from all 3 groups.
CONCLUSIONS
These findings suggest that vitamin D can attribute to the inhibition of the inflammatory response in VAT from atherosclerotic mice by reducing proinflammatory cytokine production.
Keyword
Figure
Reference
-
1. Libby P. The changing landscape of atherosclerosis. Nature. 2021; 592:524–533. PMID: 33883728.2. Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020; 16:177–189. PMID: 32020062.3. Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2014; 1842:446–462. PMID: 23707515.4. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112:1796–1808. PMID: 14679176.5. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003; 112:1821–1830. PMID: 14679177.6. Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998; 83:847–850. PMID: 9506738.7. Fain JN, Bahouth SW, Madan AK. TNFα release by the nonfat cells of human adipose tissue. Int J Obes. 2004; 28:616–622.8. Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol. 2014; 63:250–259. PMID: 24355497.9. Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010; 11:11–18. PMID: 19656312.10. Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr. 1956; 4:20–34. PMID: 13282851.11. Fantuzzi G, Mazzone T. Adipose tissue and atherosclerosis: exploring the connection. Arterioscler Thromb Vasc Biol. 2007; 27:996–1003. PMID: 17303782.12. Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019; 7:715–725. PMID: 31301983.13. Alexopoulos N, Katritsis D, Raggi P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis. 2014; 233:104–112. PMID: 24529130.14. Ohman MK, Wright AP, Wickenheiser KJ, Luo W, Eitzman DT. Visceral adipose tissue and atherosclerosis. Curr Vasc Pharmacol. 2009; 7:169–179. PMID: 19356000.15. Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010; 17:332–341. PMID: 20124732.16. Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010; 10:826–837. PMID: 21088683.17. Cole JE, Georgiou E, Monaco C. The expression and functions of toll-like receptors in atherosclerosis. Mediators Inflamm. 2010; 2010:393946. PMID: 20652007.18. Sepehri Z, Kiani Z, Nasiri AA, Kohan F. Toll-like receptor 2 and type 2 diabetes. Cell Mol Biol Lett. 2016; 21:2. PMID: 28536605.19. Wang Z, Ni X, Zhang L, Sun L, Zhu X, Zhou Q, Yang Z, Yuan H. Toll-like receptor 4 and inflammatory micro-environment of pancreatic islets in type-2 diabetes mellitus: a therapeutic perspective. Diabetes Metab Syndr Obes. 2020; 13:4261–4272. PMID: 33204132.20. Roshan MH, Tambo A, Pace NP. The role of TLR2, TLR4, and TLR9 in the pathogenesis of atherosclerosis. Int J Inflamm. 2016; 2016:1532832.21. Park CY, Han SN. The role of vitamin D in adipose tissue biology: adipocyte differentiation, energy metabolism, and inflammation. J Lipid Atheroscler. 2021; 10:130–144. PMID: 34095008.22. Ding C, Gao D, Wilding J, Trayhurn P, Bing C. Vitamin D signalling in adipose tissue. Br J Nutr. 2012; 108:1915–1923. PMID: 23046765.23. Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, Zügel U, Steinmeyer A, Pollak A, Roth E, et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006; 36:361–370. PMID: 16402404.24. Dickie LJ, Church LD, Coulthard LR, Mathews RJ, Emery P, McDermott MF. Vitamin D3 down-regulates intracellular Toll-like receptor 9 expression and Toll-like receptor 9-induced IL-6 production in human monocytes. Rheumatology (Oxford). 2010; 49:1466–1471. PMID: 20435648.25. Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012; 188:2127–2135. PMID: 22301548.26. Park CY, Kim TY, Yoo JS, Seo Y, Pae M, Han SN. Effects of 1,25-dihydroxyvitamin D3 on the inflammatory responses of stromal vascular cells and adipocytes from lean and obese mice. Nutrients. 2020; 12:12.27. Lorente-Cebrián S, Eriksson A, Dunlop T, Mejhert N, Dahlman I, Aström G, Sjölin E, Wåhlén K, Carlberg C, Laurencikiene J, et al. Differential effects of 1α,25-dihydroxycholecalciferol on MCP-1 and adiponectin production in human white adipocytes. Eur J Nutr. 2012; 51:335–342. PMID: 21701898.28. Marcotorchino J, Gouranton E, Romier B, Tourniaire F, Astier J, Malezet C, Amiot MJ, Landrier JF. Vitamin D reduces the inflammatory response and restores glucose uptake in adipocytes. Mol Nutr Food Res. 2012; 56:1771–1782. PMID: 23065818.29. Gao D, Trayhurn P, Bing C. 1,25-Dihydroxyvitamin D3 inhibits the cytokine-induced secretion of MCP-1 and reduces monocyte recruitment by human preadipocytes. Int J Obes. 2013; 37:357–365.30. Ding C, Wilding JP, Bing C. 1,25-Dihydroxyvitamin D3 protects against macrophage-induced activation of NFκB and MAPK signalling and chemokine release in human adipocytes. PLoS One. 2013; 8:e61707. PMID: 23637889.31. Karkeni E, Marcotorchino J, Tourniaire F, Astier J, Peiretti F, Darmon P, Landrier JF. Vitamin D limits chemokine expression in adipocytes and macrophage migration in vitro and in male mice. Endocrinology. 2015; 156:1782–1793. PMID: 25730105.32. Sun X, Zemel MB. Calcitriol and calcium regulate cytokine production and adipocyte-macrophage cross-talk. J Nutr Biochem. 2008; 19:392–399. PMID: 17869082.33. Sun X, Zemel MB. Calcium and 1,25-dihydroxyvitamin D3 regulation of adipokine expression. Obesity (Silver Spring). 2007; 15:340–348. PMID: 17299106.34. Surdu AM, Pinzariu O, Ciobanu DM, Negru AG, Cainap SS, Lazea C, Iacob D, Saraci G, Tirinescu D, Borda IM, et al. Vitamin D and its role in the lipid metabolism and the development of atherosclerosis. Biomedicines. 2021; 9:172. PMID: 33572397.35. Riek AE, Oh J, Bernal-Mizrachi C. 1,25(OH)2 vitamin D suppresses macrophage migration and reverses atherogenic cholesterol metabolism in type 2 diabetic patients. J Steroid Biochem Mol Biol. 2013; 136:309–312. PMID: 23333932.36. Riek AE, Oh J, Sprague JE, Timpson A, de las Fuentes L, Bernal-Mizrachi L, Schechtman KB, Bernal-Mizrachi C. Vitamin D suppression of endoplasmic reticulum stress promotes an antiatherogenic monocyte/macrophage phenotype in type 2 diabetic patients. J Biol Chem. 2012; 287:38482–38494. PMID: 23012375.37. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006; 6:772–783. PMID: 16998510.38. Coenen KR, Gruen ML, Chait A, Hasty AH. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 2007; 56:564–573. PMID: 17327423.39. Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005; 90:2282–2289. PMID: 15671098.40. Chang E. Effects of vitamin D supplementation on adipose tissue inflammation and NF-κB/AMPK activation in obese mice fed a high-fat diet. Int J Mol Sci. 2022; 23:23.41. Jiménez-Martínez M, Stamatakis K, Fresno M. The dual-specificity phosphatase 10 (DUSP10): its role in cancer, inflammation, and immunity. Int J Mol Sci. 2019; 20:1626. PMID: 30939861.42. Bijnen M, van de Gaar J, Vroomen M, Gijbels MJ, de Winther M, Schalkwijk CG, Wouters K. Adipose tissue macrophages do not affect atherosclerosis development in mice. Atherosclerosis. 2019; 281:31–37. PMID: 30654169.43. Luo LJ, Liu F, Wang XY, Dai TY, Dai YL, Dong C, Ge BX. An essential function for MKP5 in the formation of oxidized low density lipid-induced foam cells. Cell Signal. 2012; 24:1889–1898. PMID: 22683306.44. Zhang X, Zhao Z, Baldini M, Zhang C, Tao B, Zhang L, Bennett AM, Yu J. Lack of mitogen-activated kinase phosphatase-5 in macrophages protects Ldlr-null mice against atherogenesis. JVS Vasc Sci. 2022; 3:423.45. Cohen-Lahav M, Shany S, Tobvin D, Chaimovitz C, Douvdevani A. Vitamin D decreases NFκB activity by increasing IκBα levels. Nephrol Dial Transplant. 2006; 21:889–897. PMID: 16455676.46. Watanabe Y, Nagai Y, Takatsu K. Activation and regulation of the pattern recognition receptors in obesity-induced adipose tissue inflammation and insulin resistance. Nutrients. 2013; 5:3757–3778. PMID: 24064574.47. Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med (Berl). 2006; 84:712–725. PMID: 16924467.48. Ding Y, Subramanian S, Montes VN, Goodspeed L, Wang S, Han C, Teresa AS 3rd, Kim J, O’Brien KD, Chait A. Toll-like receptor 4 deficiency decreases atherosclerosis but does not protect against inflammation in obese low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2012; 32:1596–1604. PMID: 22580897.49. Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007; 26:3203–3213. PMID: 17496916.50. Pyper SR, Viswakarma N, Yu S, Reddy JK. PPARα: energy combustion, hypolipidemia, inflammation and cancer. Nucl Recept Signal. 2010; 8:e002. PMID: 20414453.51. Wu JJ, Roth RJ, Anderson EJ, Hong EG, Lee MK, Choi CS, Neufer PD, Shulman GI, Kim JK, Bennett AM. Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metab. 2006; 4:61–73. PMID: 16814733.52. Khadir A, Tiss A, Abubaker J, Abu-Farha M, Al-Khairi I, Cherian P, John J, Kavalakatt S, Warsame S, Al-Madhoun A, et al. MAP kinase phosphatase DUSP1 is overexpressed in obese humans and modulated by physical exercise. Am J Physiol Endocrinol Metab. 2015; 308:E71–E83. PMID: 25370852.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Vitamin D in Adipose Tissue Biology: Adipocyte Differentiation, Energy Metabolism, and Inflammation
- The Inhibitory Effect of Quercetin on Adipose Tissue Inflammation in Mice Fed on a High-fat Diet
- Vitamin D regulation of adipogenesis and adipose tissue functions
- Immune cells in brown adipose tissue are involved in allogeneic immune responses
- Visceral Obesity