Cancer Res Treat.
2024 Jan;56(1):324-333. 10.4143/crt.2023.738.
Impact of T-Cell Engagers on COVID-19–Related Mortality in B-Cell Lymphoma Patients Receiving B-Cell Depleting Therapy
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 2Seoul National University Cancer Research Institute, Seoul, Korea
- KMID: 2550347
- DOI: http://doi.org/10.4143/crt.2023.738
Abstract
- Purpose
B-cell depleting therapies, including T-cell engager (TCE), are increasingly used for patients with hematologic malignancies, including during the coronavirus disease 2019 (COVID-19) pandemic. We aimed to evaluate the relationship between TCE therapy and COVID-19–related outcomes among patients with COVID-19 and B-cell lymphomas receiving B-cell depleting therapy.
Materials and Methods
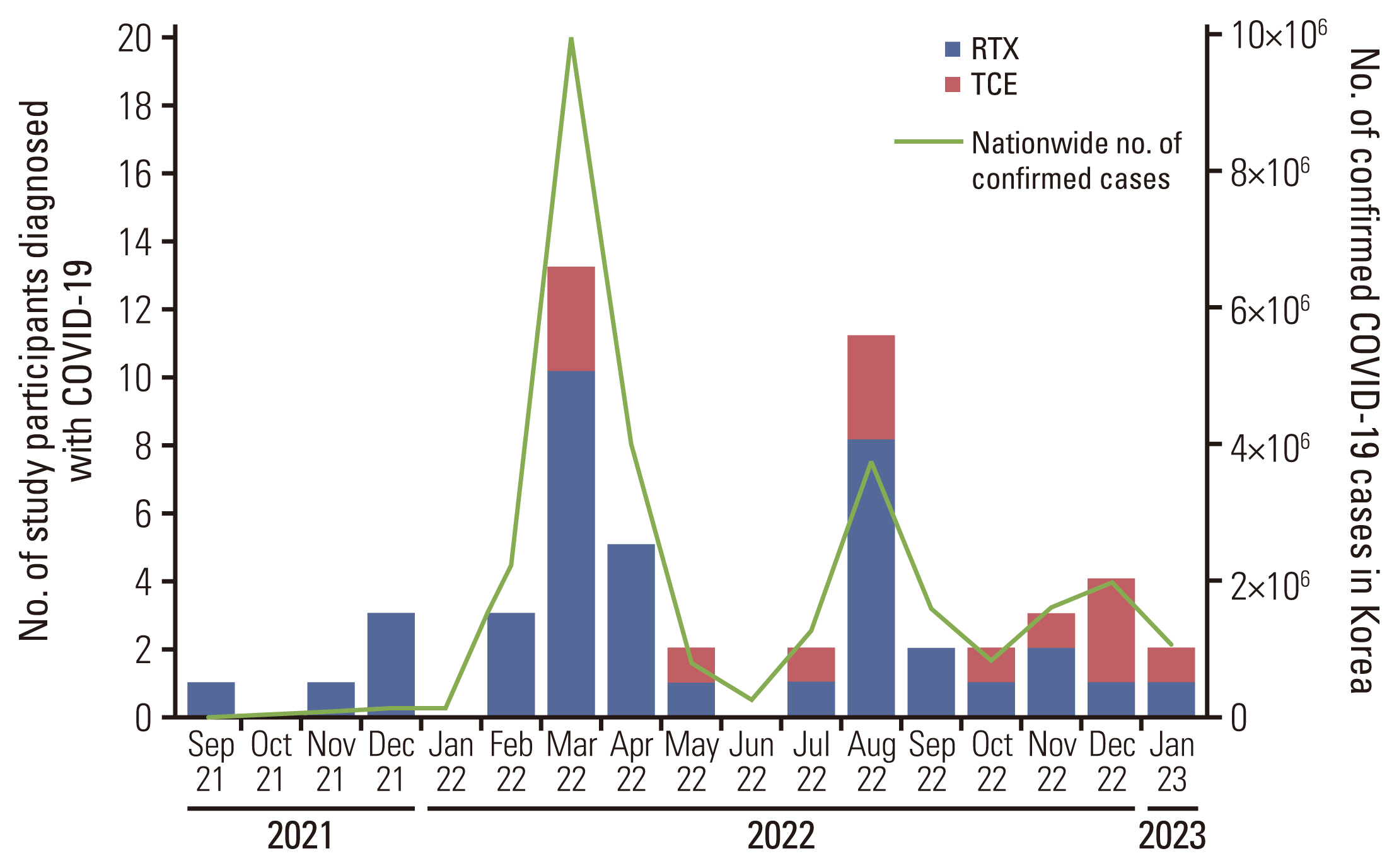
This retrospective cohort study included patients with B-cell lymphoma, who were admitted to Seoul Natio-nal University Hospital with COVID-19 between September 2021 and February 2023, and received B-cell depleting therapy before COVID-19 diagnosis. Multivariable logistic regression was used to identify factors associated with severe to critical COVID-19 and COVID-19–related mortality.
Results
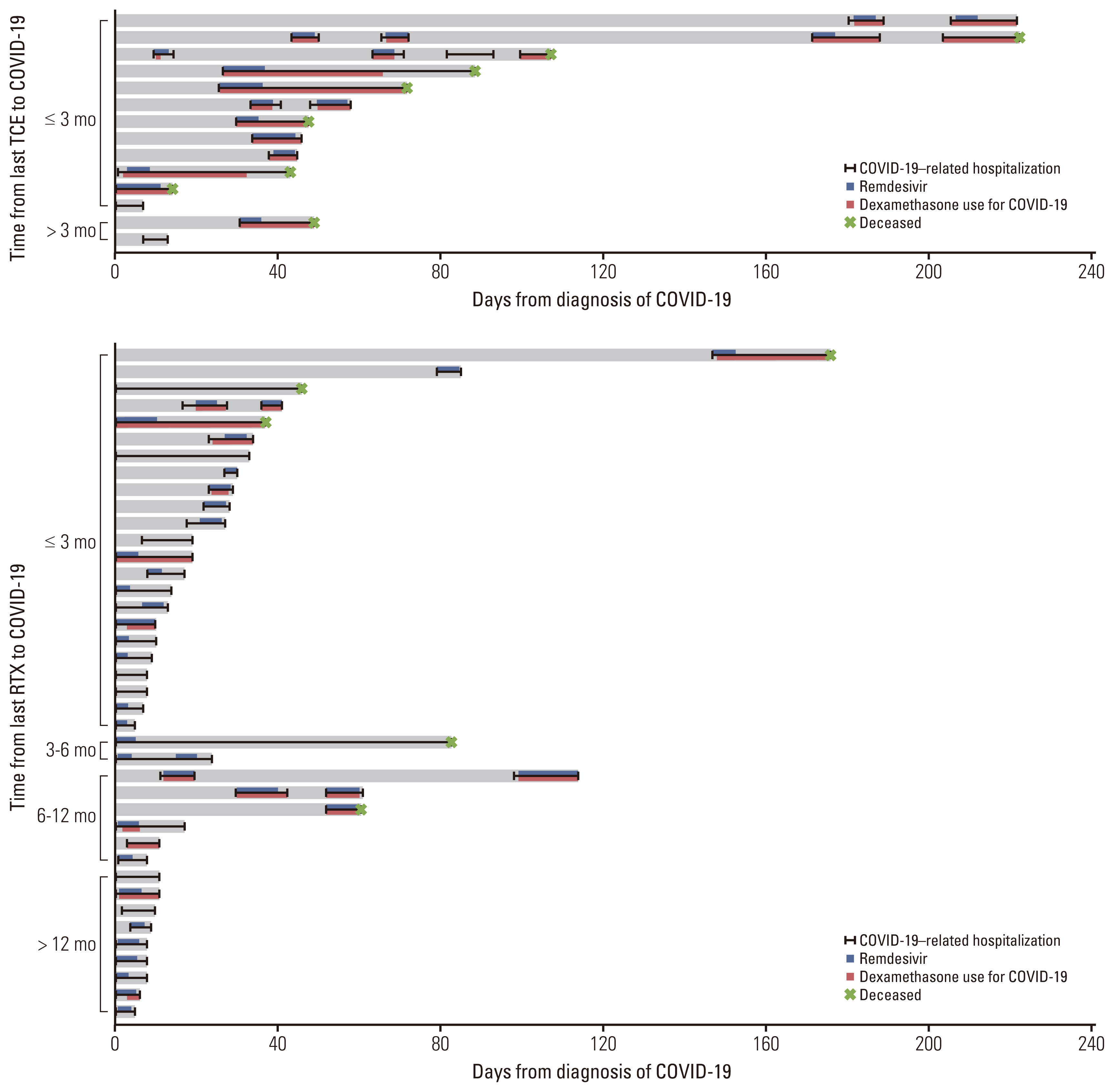
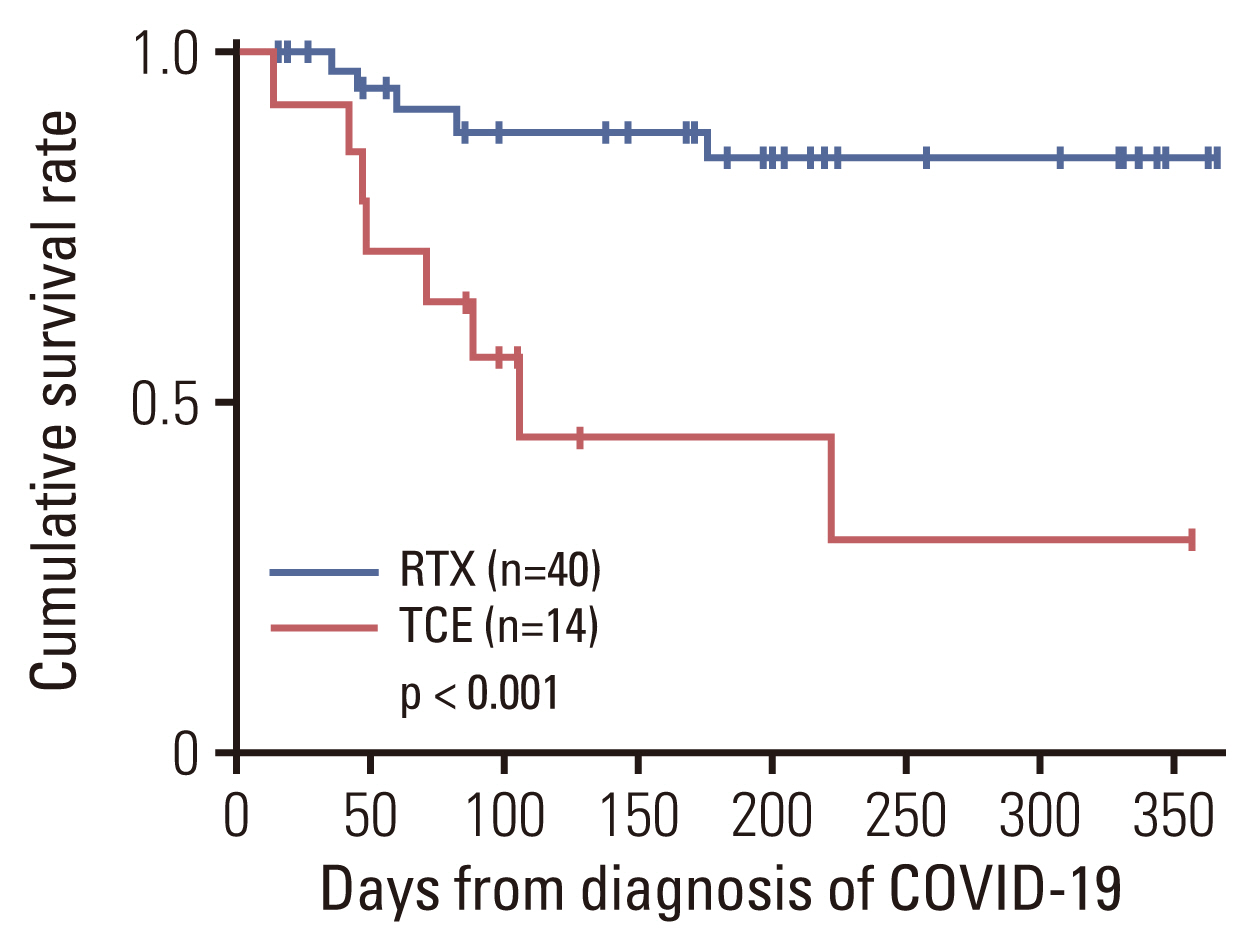
Of 54 patients with B-cell lymphomas and COVID-19 who received B-cell depleting therapy, 14 were treated with TCE (TCE group) and 40 with rituximab (RTX group). COVID-19–related mortality was higher in the TCE group than in the RTX group (57.1% vs. 12.5%, p=0.002). In multivariable analyses, TCE therapy (adjusted odds ratio [aOR], 7.08; 95% confidence interval [CI], 1.29 to 38.76; p=0.024) and older age (aOR, 1.06; 95% CI, 1.00 to 1.13; p=0.035) were associated with severe to critical COVID-19. TCE therapy (aOR, 8.98; 95% CI, 1.48 to 54.40; p=0.017), older age (aOR, 1.13; 95% CI, 1.02 to 1.26; p=0.022), and prior bendamustine therapy (aOR, 7.78; 95% CI, 1.17 to 51.65; p=0.034) were independent risk factors for COVID-19–related mortality.
Conclusion
B-cell lymphoma patients treated with TCE had significantly worse outcomes from COVID-19 than those treated with RTX. TCE therapy should be used with caution in B-cell lymphoma patients during the COVID-19 epidemic.
Keyword
Figure
Reference
-
References
1. Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021; 7:220–7.2. Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020; 7:e737–45.3. Yasuda H, Tsukune Y, Watanabe N, Sugimoto K, Uchimura A, Tateyama M, et al. Persistent COVID-19 pneumonia and failure to develop anti-SARS-CoV-2 antibodies during rituximab maintenance therapy for follicular lymphoma. Clin Lymphoma Myeloma Leuk. 2020; 20:774–6.4. Yasuda H, Mori Y, Chiba A, Bai J, Murayama G, Matsushita Y, et al. Resolution of one-year persisting COVID-19 pneumonia and development of immune thrombocytopenia in a follicular lymphoma patient with preceding rituximab maintenance therapy: a follow-up report and literature review of cases with prolonged infections. Clin Lymphoma Myeloma Leuk. 2021; 21:e810–6.5. Dulery R, Lamure S, Delord M, Di Blasi R, Chauchet A, Hueso T, et al. Prolonged in-hospital stay and higher mortality after COVID-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy. Am J Hematol. 2021; 96:934–44.6. Salles G, Barrett M, Foa R, Maurer J, O’Brien S, Valente N, et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017; 34:2232–73.7. Tian Z, Liu M, Zhang Y, Wang X. Bispecific T cell engagers: an emerging therapy for management of hematologic malignancies. J Hematol Oncol. 2021; 14:75.8. Gonzalez Barca E. Role of bispecific antibodies in relapsed/refractory diffuse large B-cell lymphoma in the CART era. Front Immunol. 2022; 13:909008.9. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines [Internet]. Bethesda, MD: National Institutes of Health;2022 [cited 2023 Mar 21]. Available from: http://www.covid19treatmentguidelines.nih.gov/ .10. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127:2375–90.11. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32:3059–68.12. Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022; 399:1303–12.13. Perez P, Hoffman RW, Shaw S, Bluestone JA, Segal DM. Specific targeting of cytotoxic T cells by anti-T3 linked to anti-target cell antibody. Nature. 1985; 316:354–6.14. Schuster SJ. Bispecific antibodies for the treatment of lymphomas: promises and challenges. Hematol Oncol. 2021; 39 (Suppl 1):113–6.15. Falchi L, Vardhana SA, Salles GA. Bispecific antibodies for the treatment of B-cell lymphoma: promises, unknowns, and opportunities. Blood. 2023; 141:467–80.16. Dickinson MJ, Carlo-Stella C, Morschhauser F, Bachy E, Corradini P, Iacoboni G, et al. Glofitamab for relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2022; 387:2220–31.17. Budde LE, Sehn LH, Matasar M, Schuster SJ, Assouline S, Giri P, et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol. 2022; 23:1055–65.18. Hutchings M, Mous R, Clausen MR, Johnson P, Linton KM, Chamuleau MED, et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet. 2021; 398:1157–69.19. Budde LE, Assouline S, Sehn LH, Schuster SJ, Yoon SS, Yoon DH, et al. Single-agent mosunetuzumab shows durable complete responses in patients with relapsed or refractory B-cell lymphomas: phase I dose-escalation study. J Clin Oncol. 2022; 40:481–91.20. Hutchings M, Morschhauser F, Iacoboni G, Carlo-Stella C, Offner FC, Sureda A, et al. Glofitamab, a novel, bivalent CD20-targeting T-cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: a phase I trial. J Clin Oncol. 2021; 39:1959–70.21. Bannerji R, Arnason JE, Advani RH, Brown JR, Allan JN, Ansell SM, et al. Odronextamab, a human CD20xCD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022; 9:e327–39.22. Thieblemont C, Phillips T, Ghesquieres H, Cheah CY, Clausen MR, Cunningham D, et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell-engaging antibody, in relapsed or refractory large B-cell lymphoma: dose expansion in a phase I/II trial. J Clin Oncol. 2023; 41:2238–47.23. Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. 2013; 13:106–11.24. Aksoy S, Dizdar O, Hayran M, Harputluoglu H. Infectious complications of rituximab in patients with lymphoma during maintenance therapy: a systematic review and meta-analysis. Leuk Lymphoma. 2009; 50:357–65.25. Garcia-Suarez J, de la Cruz J, Cedillo A, Llamas P, Duarte R, Jimenez-Yuste V, et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. J Hematol Oncol. 2020; 13:133.26. O’Leary AL, Wattengel BA, Carter MT, Drye AF, Mergenhagen KA. Risk factors associated with mortality in hospitalized patients with laboratory confirmed SARS-CoV-2 infection during the period of omicron (B.1.1.529) variant predominance. Am J Infect Control. 2023; 51:603–6.27. Assanto GM, Di Rocco A, Malfona F, Capriata M, Del Giudice I, Petrucci L, et al. Impact of anti-SARS-CoV-2 monoclonal antibodies in the management of patients with lymphoma and COVID19: a retrospective study. Hematol Oncol. 2023; 41:343–53.28. Hagihara M, Imai Y, Uchida T, Ohara S, Inoue M, Sugi T, et al. Successful elimination of SARS-CoV-2 following vaccination with BNT162b2 after prolonged viral infection in an immunocompromised lymphoma patient. Intern Med. 2022; 61:2215–9.29. Nakamura S, Kanemasa Y, Atsuta Y, Fujiwara S, Tanaka M, Fukushima K, et al. Characteristics and outcomes of coronavirus disease 2019 (COVID-19) patients with cancer: a single-center retrospective observational study in Tokyo, Japan. Int J Clin Oncol. 2021; 26:485–93.30. Fung M, Jacobsen E, Freedman A, Prestes D, Farmakiotis D, Gu X, et al. Increased risk of infectious complications in older patients with indolent non-Hodgkin lymphoma exposed to bendamustine. Clin Infect Dis. 2019; 68:247–55.31. Anolik JH, Friedberg JW, Zheng B, Barnard J, Owen T, Cushing E, et al. B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clin Immunol. 2007; 122:139–45.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Migratory Pneumonia in Prolonged SARS-CoV-2 Infection in Patients Treated With B-cell Depletion Therapies for B-cell Lymphoma
- The impact of COVID-19 on acute myeloid leukemia patients undergoing allogeneic stem cell transplantation: a concise review
- A Case of Extranodall NK/T-cell Lymphoma, Nasal type
- Immunohistochemical Classification and Clinical Evaluation of Nasal Malignant Lymphoma
- Primary Cutaneous T-cell/histiocyte-rich B-cell Lymphoma