Cancer Res Treat.
2024 Jan;56(1):208-218. 10.4143/crt.2022.1328.
First-in-Human Phase 1 Study of a B Cell– and Monocyte-Based Immunotherapeutic Vaccine against HER2-Positive Advanced Gastric Cancer
- Affiliations
-
- 1Division of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Center, Yonsei University of College of Medicine, Seoul, Korea
- 2Song-dang Institute for Cancer Research, Yonsei University College of Medicine, Seoul, Korea
- 3Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea
- 4Cellid, Inc., Seoul, Korea
- 5Laboratory of Immunology, College of Pharmacy, Seoul National University, Seoul, Korea
- 6Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2550337
- DOI: http://doi.org/10.4143/crt.2022.1328
Abstract
- Purpose
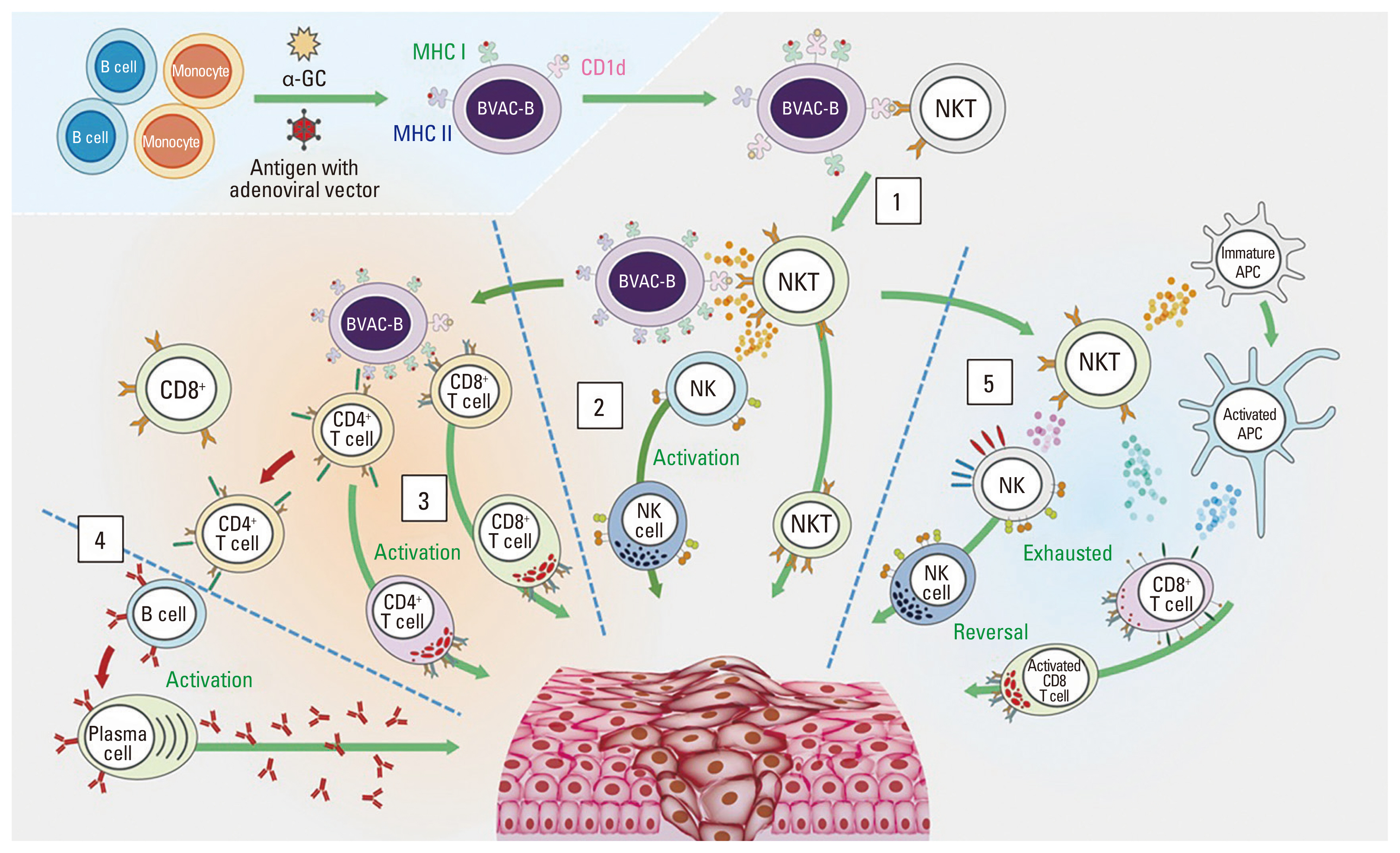
BVAC-B is an autologous B cell– and monocyte-based immunotherapeutic vaccine that contains cells transfected with a recombinant human epidermal growth factor receptor 2 (HER2) gene and loaded with the natural killer T cell ligand alpha-galactosylceramide. Here, we report the first BVAC-B study in patients with HER2-positive advanced gastric cancer.
Materials and Methods
Patients with advanced gastric cancer refractory to standard treatment with HER2+ immunohistochemistry ≥ 1 were eligible for treatment. Patients were administered low (2.5×107 cells/dose), medium (5.0×107 cells/dose), or high dose (1.0×108 cells/dose) of BVAC-B intravenously four times every 4 weeks. Primary endpoints included safety and maximum tolerated BVAC-B dose. Secondary endpoints included preliminary clinical efficacy and BVAC-B-induced immune responses.
Results
Eight patients were treated with BVAC-B at low (n=1), medium (n=1), and high doses (n=6). No dose-limiting toxicity was observed, while treatment-related adverse events (TRAEs) were observed in patients treated with medium and high doses. The most common TRAEs were grade 1 (n=2) and grade 2 (n=2) fever. Out of the six patients treated with high-dose BVAC-B, three had stable disease with no response. Interferon gamma, tumor necrosis factor-α, and interleukin-6 increased after BVAC-B treatment in all patients with medium and high dose, and HER2-specific antibody was detected in some patients.
Conclusion
BVAC-B monotherapy had a safe toxicity profile with limited clinical activity; however, it activated immune cells in heavily pretreated patients with HER2-positive gastric cancer. Earlier treatment with BVAC-B and combination therapy is warranted for evaluation of clinical efficacy.
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.2. Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008; 19:1523–9.3. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–97.4. Li W, Zhang X, Du Y, Zhang Y, Lu J, Hu W, et al. HER2-targeted advanced metastatic gastric/gastroesophageal junction adenocarcinoma: treatment landscape and future perspectives. Biomark Res. 2022; 10:71.5. Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020; 382:2419–30.6. Mitani S, Kawakami H. Emerging targeted therapies for HER2 positive gastric cancer that can overcome trastuzumab resistance. Cancers (Basel). 2020; 12:400.7. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018; 4:e180013.8. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017; 390:2461–71.9. Moehler M, Shitara K, Garrido M, Salman P, Shen L, Wyrwicz L, et al. LBA6_PR nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): first results of the CheckMate 649 study. Ann Oncol. 2020; 31(Suppl 4):S1191.10. Janjigian YY, Maron SB, Chatila WK, Millang B, Chavan SS, Alterman C, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020; 21:821–31.11. Janjigian YY, Kawazoe A, Yanez P, Li N, Lonardi S, Kolesnik O, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021; 600:727–30.12. Yang L, Wang Y, Wang H. Use of immunotherapy in the treatment of gastric cancer. Oncol Lett. 2019; 18:5681–90.13. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017; 377:2531–44.14. Ma S, Li X, Wang X, Cheng L, Li Z, Zhang C, et al. Current progress in CAR-T cell therapy for solid tumors. Int J Biol Sci. 2019; 15:2548–60.15. Ko HJ, Lee JM, Kim YJ, Kim YS, Lee KA, Kang CY. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. J Immunol. 2009; 182:1818–28.16. Choi CH, Choi HJ, Lee JW, Kang ES, Cho D, Park BK, et al. Phase I study of a B cell-based and monocyte-based immunotherapeutic vaccine, BVAC-C in human papillomavirus type 16- or 18-positive recurrent cervical cancer. J Clin Med. 2020; 9:147.17. Kim EK, Seo HS, Chae MJ, Jeon IS, Song BY, Park YJ, et al. Enhanced antitumor immunotherapeutic effect of B-cell-based vaccine transduced with modified adenoviral vector containing type 35 fiber structures. Gene Ther. 2014; 21:106–14.18. Lee JB, Kwon WS, Kim HS, Jung M, Kim S, Park M, et al. First-in-human phase I study of BVAC-B cell therapy in HER2-positive advanced gastric cancer. J Clin Oncol. 2020; 38(15 Suppl):4534.19. Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997; 89:1138–47.20. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.03. Bethesda, MD: National Cancer Institute;2010.21. Martinez M, Moon EK. CAR T cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol. 2019; 10:128.22. Chavez JC, Bachmeier C, Kharfan-Dabaja MA. CAR T-cell therapy for B-cell lymphomas: clinical trial results of available products. Ther Adv Hematol. 2019; 10:2040620719841581.23. Newick K, O’Brien S, Moon E, Albelda SM. CAR T cell therapy for solid tumors. Annu Rev Med. 2017; 68:139–52.24. Rahma OE, Khleif SN. Therapeutic vaccines for gastrointestinal cancers. Gastroenterol Hepatol (N Y). 2011; 7:517–64.25. Sundar R, Rha SY, Yamaue H, Katsuda M, Kono K, Kim HS, et al. A phase I/Ib study of OTSGC-A24 combined peptide vaccine in advanced gastric cancer. BMC Cancer. 2018; 18:332.26. Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004; 10:909–15.27. Nicolas-Morales ML, Luisa-Sanjuan A, Gutierrez-Torres M, Vences-Velazquez A, Ortuno-Pineda C, Espinoza-Rojo M, et al. Peptide-based vaccines in clinical phases and new potential therapeutic targets as a new approach for breast cancer: a review. Vaccines (Basel). 2022; 10:1249.28. Mittendorf EA, Lu B, Melisko M, Price Hiller J, Bondarenko I, Brunt AM, et al. Efficacy and safety analysis of Nelipepimut-S vaccine to prevent breast cancer recurrence: a randomized, multicenter, phase III clinical trial. Clin Cancer Res. 2019; 25:4248–54.29. Seo S, Ryu MH, Park YS, Ahn JY, Park Y, Park SR, et al. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: results of the GASTric cancer HER2 reassessment study 3 (GASTHER3). Gastric Cancer. 2019; 22:527–35.30. Tanaka H, Tsunoda T, Nukaya I, Sette A, Matsuda K, Umano Y, et al. Mapping the HLA-A24-restricted T-cell epitope peptide from a tumour-associated antigen HER2/neu: possible immunotherapy for colorectal carcinomas. Br J Cancer. 2001; 84:94–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- GASTric Cancer HER2 Re-Assessment Study 2 (GASTHER2): HER2 Re-assessment for Initially HER2-Negative Advanced Gastric Cancer Patients after Progression on First-Line Treatment
- Breakthroughs in the Systemic Treatment of HER2-Positive Advanced/Metastatic Gastric Cancer: From Singlet Chemotherapy to Triple Combination
- Correlation between HER2 Overexpression and Clinicopathological Characteristics in Gastric Cancer Patients Who Have Undergone Curative Resection
- Therapeutic Vaccine for Lymphoma
- Current Approaches in Development of Immunotherapeutic Vaccines for Breast Cancer