Cancer Res Treat.
2023 Oct;55(4):1104-1112. 10.4143/crt.2023.502.
Phase II Trial of Combined Durvalumab Plus Tremelimumab with Proton Therapy for Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Division of Radiation-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2547785
- DOI: http://doi.org/10.4143/crt.2023.502
Abstract
- Purpose
This phase II study investigated whether durvalumab/tremelimumab with proton therapy improves the objective response rate (ORR), overall survival (OS), and progression-free survival (PFS) in heavily treated recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) patients.
Materials and Methods
Patients who previously received more than one chemotherapy, including at least one platinum-based regimen, and who had at least two measurable lesions were enrolled. Patients received 1,500 mg durvalumab intravenously combined with 75 mg tremelimumab intravenously every 4 weeks for four cycles followed by 1,500 mg durvalumab every 4 weeks. After one cycle of the durvalumab/tremelimumab treatment, proton therapy was given with a total dose of 25 Gy in 5 Gy daily fractions to one of the measurable lesions. We also assessed the ORR in the target lesion outside the radiation field to evaluate the abscopal effect.
Results
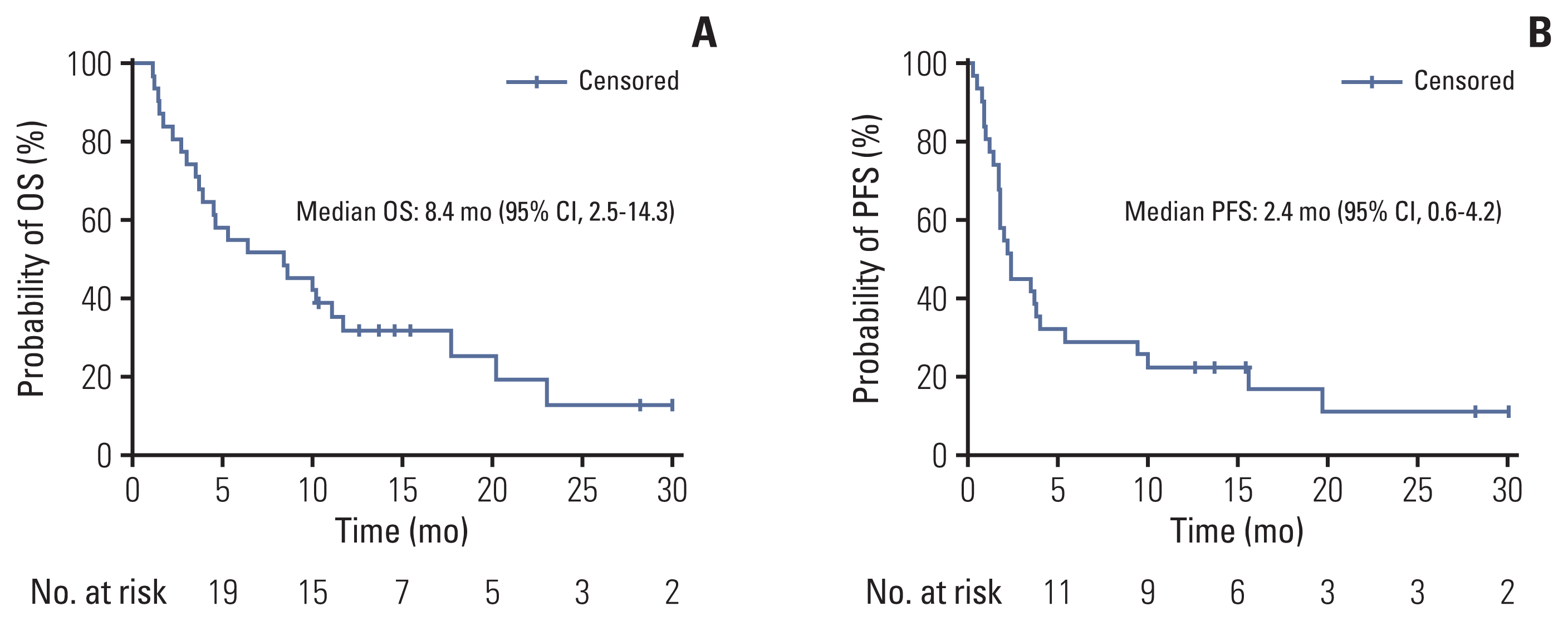
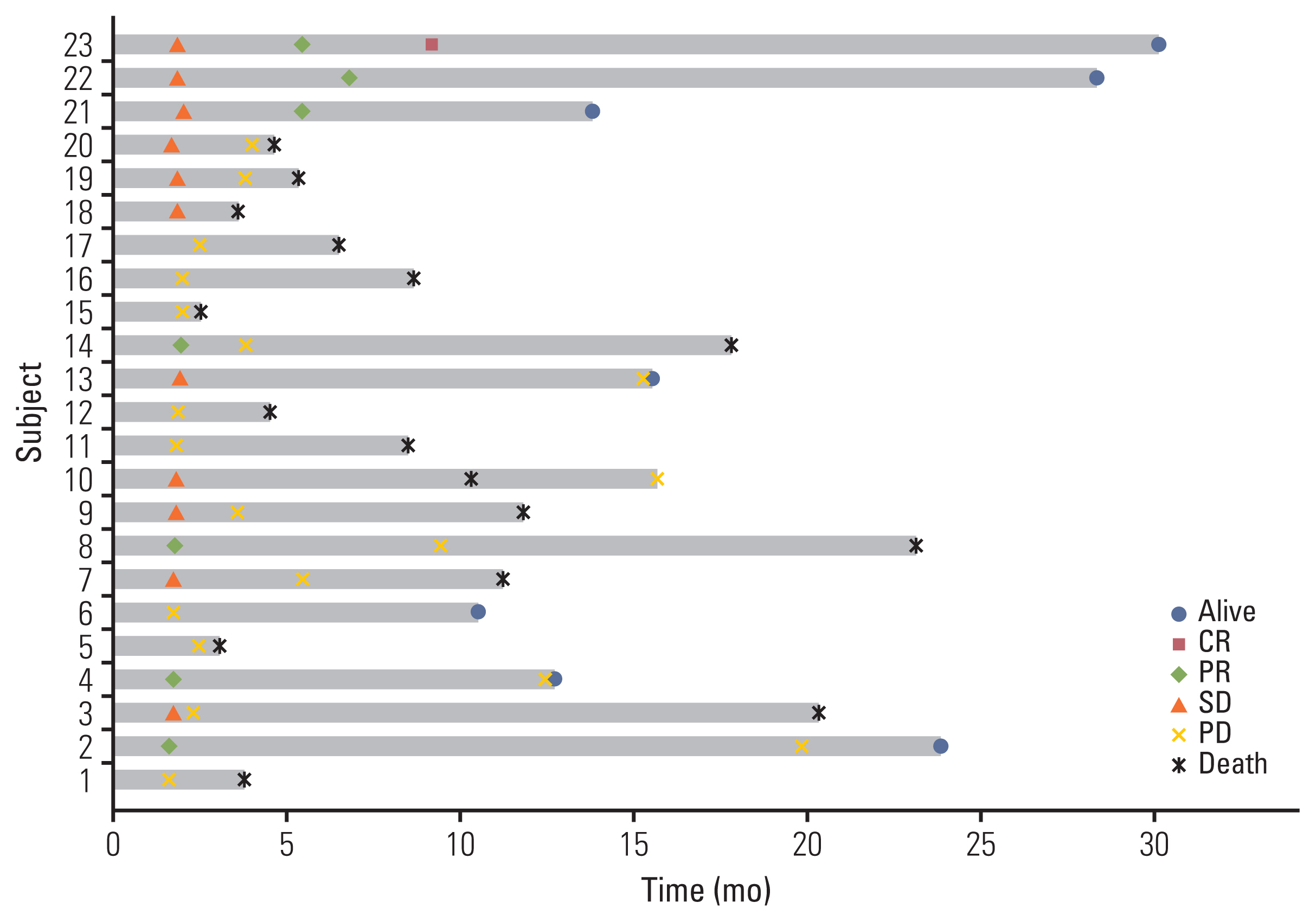
Thirty-one patients were enrolled between March 2018 and July 2020. With 8.6 months of follow-up, the ORR was 22.6% (7/31), including one complete response and six partial responses. The median OS was 8.4 months (95% confidence interval [CI], 2.5 to 14.3) and the median PFS was 2.4 months (95% CI, 0.6 to 4.2). Among the 23 evaluable patients who completed proton therapy, the ORR was 30.4% (7/23). The median OS was 11.1 months (95% CI, 6.5 to 15.8), and the median PFS was 3.7 months (95% CI, 1.6 to 5.7). Grade 3 or higher adverse events were observed in six patients (19.4%) as follows: anemia (n=1), constipation (n=1), electrolyte imbalances (n=2), hyperglycemia (n=1), and pneumonia (n=1).
Conclusion
The combination of durvalumab/tremelimuab with proton therapy was tolerated well and had encouraging anti-tumor efficacy in non-irradiated tumor lesions of heavily treated HNSCC patients.
Keyword
Figure
Reference
-
References
1. Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol. 2010; 21:Suppl 7. vii252–61.2. Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008; 359:1116–27.3. Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G Jr, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019; 394:1915–28.4. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016; 375:1856–67.5. Cohen EE, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019; 393:156–67.6. Burtness B, Rischin D, Greil R, Soulieres D, Tahara M, de Castro G Jr, et al. Pembrolizumab alone or with chemotherapy for recurrent/metastatic head and neck squamous cell carcinoma in KEYNOTE-048: subgroup analysis by programmed death ligand-1 combined positive score. J Clin Oncol. 2022; 40:2321–32.7. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018; 81:45–51.8. Stewart R, Morrow M, Hammond SA, Mulgrew K, Marcus D, Poon E, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res. 2015; 3:1052–62.9. Tarhini AA, Kirkwood JM. Tremelimumab (CP-675,206): a fully human anticytotoxic T lymphocyte-associated antigen 4 monoclonal antibody for treatment of patients with advanced cancers. Expert Opin Biol Ther. 2008; 8:1583–93.10. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12:252–64.11. Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016; 17:299–308.12. Ferris RL, Haddad R, Even C, Tahara M, Dvorkin M, Ciuleanu TE, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol. 2020; 31:942–50.13. Siu LL, Even C, Mesia R, Remenar E, Daste A, Delord JP, et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol. 2019; 5:195–203.14. Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009; 10:718–26.15. Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009; 15:5379–88.16. Mole RH. Whole body irradiation: radiobiology or medicine? Br J Radiol. 1953; 26. 305:234–41.17. Gerweck LE, Kozin SV. Relative biological effectiveness of proton beams in clinical therapy. Radiother Oncol. 1999; 50:135–42.18. Davuluri R, Jiang W, Fang P, Xu C, Komaki R, Gomez DR, et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2017; 99:128–35.19. Lee HJ Jr., Zeng J, Rengan R. Proton beam therapy and immunotherapy: an emerging partnership for immune activation in non-small cell lung cancer. Transl Lung Cancer Res. 2018; 7:180–8.20. Zhuang H. Abscopal effect of stereotactic radiotherapy combined with anti-PD-1/PD-L1 immunotherapy: mechanisms, clinical efficacy, and issues. Cancer Commun (Lond). 2020; 40:649–54.21. Brenneman RJ, Sharifai N, Fischer-Valuck B, Hassanzadeh C, Guzelian J, Chrisinger JS, et al. Abscopal effect following proton beam radiotherapy in a patient with inoperable metastatic retroperitoneal sarcoma. Front Oncol. 2019; 9:922.22. Wang DD, Zhang S, Zhao H, Men AY, Parivar K. Fixed dosing versus body size-based dosing of monoclonal antibodies in adult clinical trials. J Clin Pharmacol. 2009; 49:1012–24.23. Goto T. Radiation as an in situ auto-vaccination: current perspectives and challenges. Vaccines (Basel). 2019; 7:100.24. Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC: update from PACIFIC. J Thorac Oncol. 2020; 15:288–93.25. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018; 379:2342–50.26. Theelen W, Chen D, Verma V, Hobbs BP, Peulen HM, Aerts J, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. 2021; 9:467–75.27. Bestvina CM, Pointer KB, Karrison T, Al-Hallaq H, Hoffman PC, Jelinek MJ, et al. A phase 1 trial of concurrent or sequential ipilimumab, nivolumab, and stereotactic body radiotherapy in patients with stage IV NSCLC study. J Thorac Oncol. 2022; 17:130–40.28. McBride S, Sherman E, Tsai CJ, Baxi S, Aghalar J, Eng J, et al. Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J Clin Oncol. 2021; 39:30–7.29. Nizam A, Aragon-Ching JB. Frontline immunotherapy treatment with nivolumab and ipilimumab in metastatic renal cell cancer: a new standard of care. Cancer Biol Ther. 2019; 20:6–7.30. Reck M, Borghaei H, O’Byrne KJ. Nivolumab plus ipilimumab in non-small-cell lung cancer. Future Oncol. 2019; 15:2287–302.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A phase II study of neoadjuvant chemotherapy plus durvalumab and tremelimumab in advanced-stage ovarian cancer: a Korean Gynecologic Oncology Group Study (KGOG 3046), TRU-D
- EGFR-targeted Therapy in Head and Neck Squamous Cell Carcinoma
- Herpes Viral Gene Therapy for the Treatment of Head and Neck Squamous Cell Carcinoma
- The value of salvage operation for recurrent head and neck cancer after surgery alone or surgery with radiotherapy
- Immunotherapy in Head and Neck Squamous Cell Cancer