Nutr Res Pract.
2023 Oct;17(5):899-916. 10.4162/nrp.2023.17.5.899.
Neuroprotective effects of hesperetin on H 2 O 2 -induced damage in neuroblastoma SH-SY5Y cells
- Affiliations
-
- 1Department of Food and Nutrition, Chonnam National University, Gwangju 61186, Korea
- KMID: 2546947
- DOI: http://doi.org/10.4162/nrp.2023.17.5.899
Abstract
- BACKGROUND/OBJECTIVES
Oxidative stress is a fundamental neurodegenerative disease trigger that damages and decimates nerve cells. Neurodegenerative diseases are chronic central nervous system disorders that progress and result from neuronal degradation and loss. Recent studies have extensively focused on neurodegenerative disease treatment and prevention using dietary compounds. Heseperetin is an aglycone hesperidin form with various physiological activities, such as anti-inflammation, antioxidant, and antitumor. However, few studies have considered hesperetin’s neuroprotective effects and mechanisms; thus, our study investigated this in hydrogen peroxide ( H 2 O 2 )-treated SH-SY5Y cells.
MATERIALS/METHODS
SH-SY5Y cells were treated with H 2 O 2 (400 µM) in hesperetin absence or presence (10–40 µM) for 24 h. Three-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide assays detected cell viability, and 4′,6-diamidino-2-phenylindole staining allowed us to observe nuclear morphology changes such as chromatin condensation and apoptotic nuclei. Reactive oxygen species (ROS) detection assays measured intracellular ROS production; Griess reaction assays assessed nitric oxide (NO) production. Western blotting and quantitative polymerase chain reactions quantified corresponding mRNA and proteins.
RESULTS
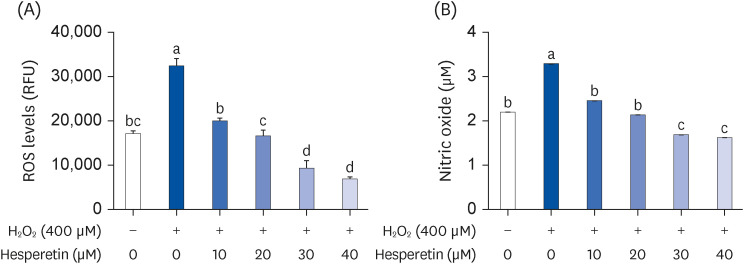
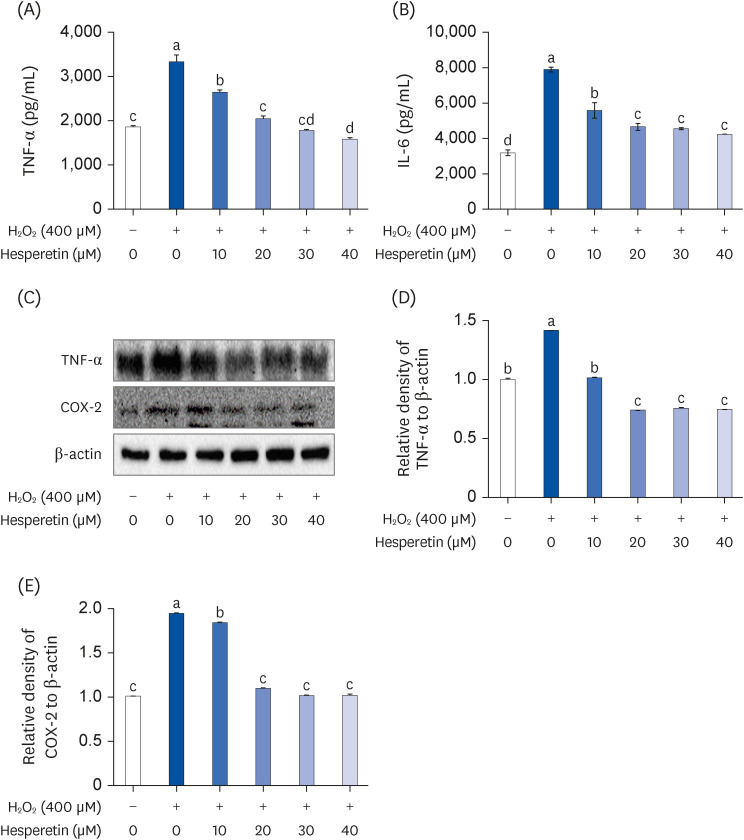
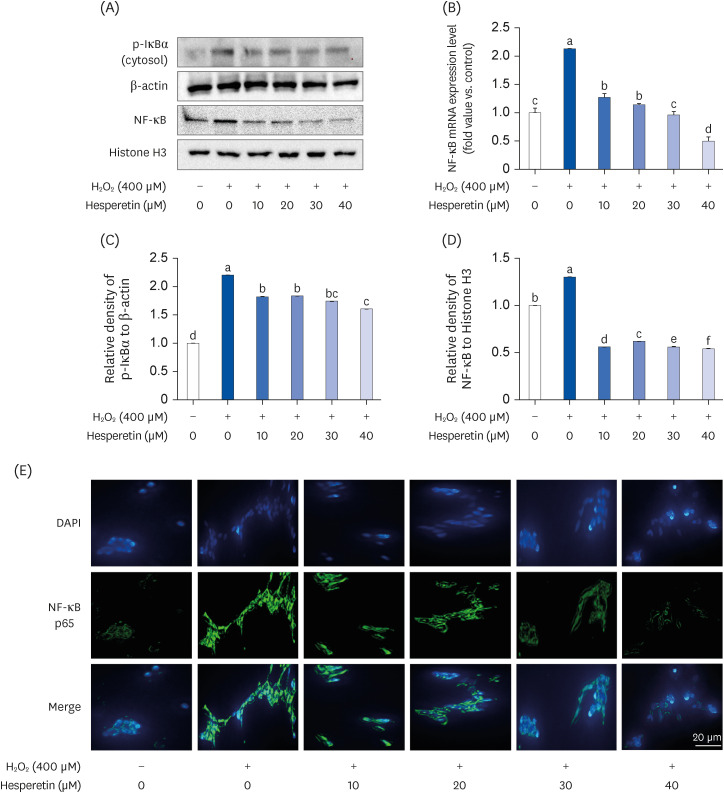
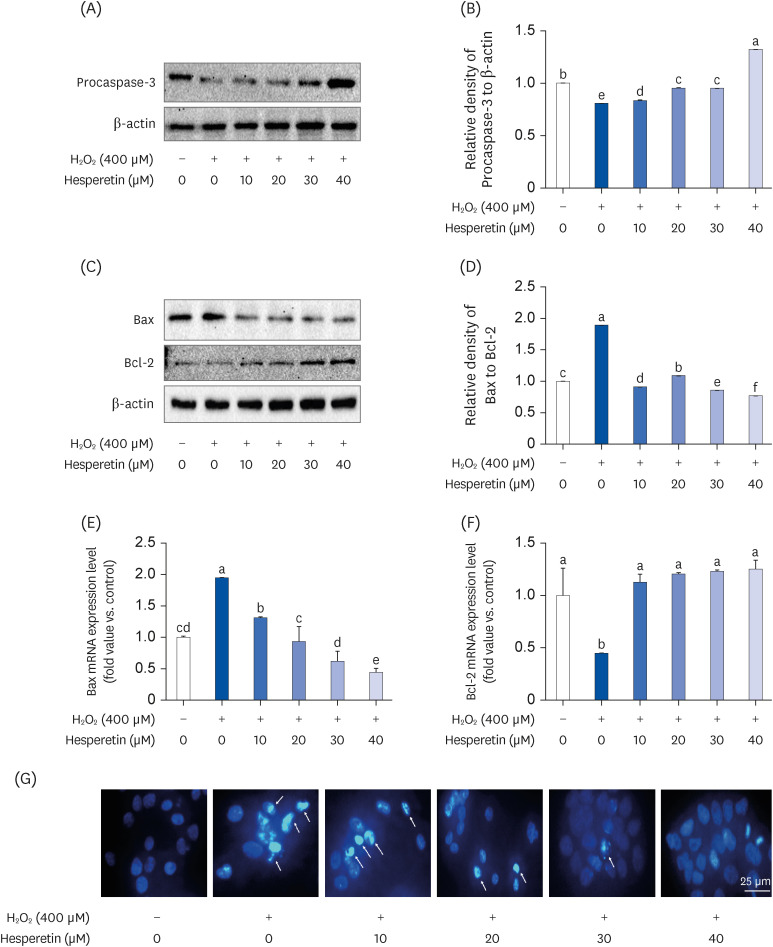
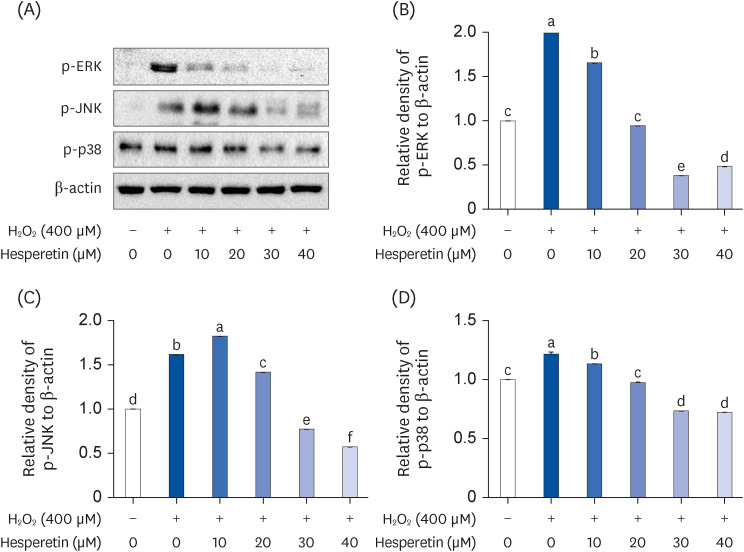
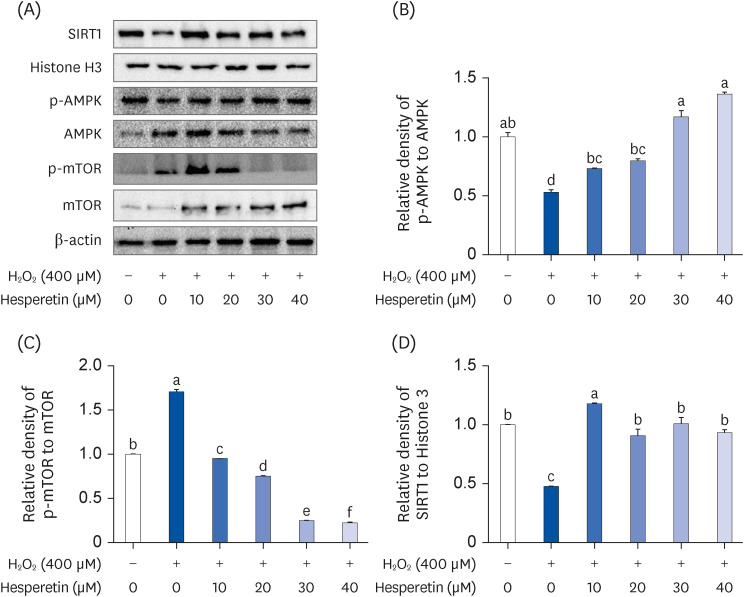
Subsequent experiments utilized various non-toxic hesperetin concentrations, establishing that hesperetin notably decreased intracellular ROS and NO production in H 2 O 2 -treated SH-SY5Y cells (P < 0.05). Furthermore, hesperetin inhibited H 2 O 2 -induced inflammation-related gene expression, including interluekin-6, tumor necrosis factor-α, and nuclear factor kappa B (NF-κB) p65 activation. In addition, hesperetin inhibited NFκB translocation into H 2 O 2 -treated SH-SY5Y cell nuclei and suppressed mitogen-activated protein kinase protein expression, an essential apoptotic cell death regulator. Various apoptosis hallmarks, including shrinkage and nuclear condensation in H 2 O 2 -treated cells, were suppressed dose-dependently. Additionally, hesperetin treatment down-regulated Bax/ Bcl-2 expression ratios and activated AMP-activated protein kinase-mammalian target of rapamycin autophagy pathways.
CONCLUSION
These results substantiate that hesperetin activates autophagy and inhibits apoptosis and inflammation. Hesperetin is a potentially potent dietary agent that reduces neurodegenerative disease onset, progression, and prevention
Figure
Reference
-
1. Singh A, Kukreti R, Saso L, Kukreti S. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules. 2019; 24:1583. PMID: 31013638.2. Simonian NA, Coyle JT. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 1996; 36:83–106. PMID: 8725383.3. Sharifi-Rad M, Anil Kumar NV, Zucca P, Varoni EM, Dini L, Panzarini E, Rajkovic J, Tsouh Fokou PV, Azzini E, Peluso I, et al. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol. 2020; 11:694. PMID: 32714204.4. Whittemore ER, Loo DT, Watt JA, Cotman CW. A detailed analysis of hydrogen peroxide-induced cell death in primary neuronal culture. Neuroscience. 1995; 67:921–932. PMID: 7675214.5. Lindenboim L, Haviv R, Stein R. Bcl-xL inhibits different apoptotic pathways in rat PC12 cells. Neurosci Lett. 1998; 253:37–40. PMID: 9754799.6. Rojkind M, Domínguez-Rosales JA, Nieto N, Greenwel P. Role of hydrogen peroxide and oxidative stress in healing responses. Cell Mol Life Sci. 2002; 59:1872–1891. PMID: 12530519.7. Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010; 129:154–169. PMID: 20561356.8. Hassanzadeh-Taheri M, Ahmadi-Zohan A, Mohammadifard M, Hosseini M. Rosmarinic acid attenuates lipopolysaccharide-induced neuroinflammation and cognitive impairment in rats. J Chem Neuroanat. 2021; 117:102008. PMID: 34314849.9. Ha SK, Moon E, Ju MS, Kim DH, Ryu JH, Oh MS, Kim SY. 6-Shogaol, a ginger product, modulates neuroinflammation: a new approach to neuroprotection. Neuropharmacology. 2012; 63:211–223. PMID: 22465818.10. Bae MK, Choi S, Ko MJ, Ha HJ, Kim HJ. Effect of OQ21 and melatonin on lipopolysaccharide-induced oxidative stress in rat brain. Yakhak Hoeji. 2005; 49:347–354.11. Choi WH, Oh YS, Kim SR, Ahn JY, Ha TY. Antioxidative and protective effects of Ulmus davidiana var. japonica extracts on glutamate-induced cytotoxicity in PC 12 cells. Korean J Food Sci Technol. 2005; 37:479–483.12. Good PF, Werner P, Hsu A, Olanow CW, Perl DP. Evidence of neuronal oxidative damage in Alzheimer’s disease. Am J Pathol. 1996; 149:21–28. PMID: 8686745.13. Radi E, Formichi P, Battisti C, Federico A. Apoptosis and oxidative stress in neurodegenerative diseases. J Alzheimers Dis. 2014; 42(Suppl 3):S125–S152. PMID: 25056458.14. Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000; 45:528–537. PMID: 10728374.15. Kim JH, Yang HK, Hong HJ, Kang WY, Kim DG, Kim SC, Song KJ, King D, Han CH, et al. Neuroprotective effects of Korean kiwifruit against t-BHP-induced cell damage in PC12 cells. Korean J Plant Res. 2010; 23:165–171.16. Chen B, Yue R, Yang Y, Zeng H, Chang W, Gao N, Yuan X, Zhang W, Shan L. Protective effects of (E)-2-(1-hydroxyl-4-oxocyclohexyl) ethyl caffeine against hydrogen peroxide-induced injury in PC12 cells. Neurochem Res. 2015; 40:531–541. PMID: 25503480.17. Tian X, Guo LP, Hu XL, Huang J, Fan YH, Ren TS, Zhao QC. Protective effects of Arctium lappa L. roots against hydrogen peroxide-induced cell injury and potential mechanisms in SH-SY5Y cells. Cell Mol Neurobiol. 2015; 35:335–344. PMID: 25352420.18. Chang KC, Liu PF, Chang CH, Lin YC, Chen YJ, Shu CW. The interplay of autophagy and oxidative stress in the pathogenesis and therapy of retinal degenerative diseases. Cell Biosci. 2022; 12:1. PMID: 34980273.19. Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013; 19:983–997. PMID: 23921753.20. Tong W, Ju L, Qiu M, Xie Q, Chen Y, Shen W, Sun W, Wang W, Tian J. Liraglutide ameliorates non-alcoholic fatty liver disease by enhancing mitochondrial architecture and promoting autophagy through the SIRT1/SIRT3-FOXO3a pathway. Hepatol Res. 2016; 46:933–943. PMID: 26666995.21. Yang L, Shi J, Wang X, Zhang R. Curcumin alleviates D-galactose-induced cardiomyocyte senescence by promoting autophagy via the SIRT1/AMPK/mTOR pathway. Evid Based Complement Alternat Med. 2022; 2022:2990843. PMID: 35880107.22. Park SK, Seong RK, Kim JA, Son SJ, Kim Y, Yokozawa T, Shin OS. Oligonol promotes anti-aging pathways via modulation of SIRT1-AMPK-Autophagy Pathway. Nutr Res Pract. 2016; 10:3–10. PMID: 26865910.23. Bockaert J, Marin P. mTOR in brain physiology and pathologies. Physiol Rev. 2015; 95:1157–1187. PMID: 26269525.24. Carosi JM, Sargeant TJ. Rapamycin and Alzheimer disease: a double-edged sword? Autophagy. 2019; 15:1460–1462. PMID: 31066320.25. Velmurugan BK, Rathinasamy B, Lohanathan BP, Thiyagarajan V, Weng CF. Neuroprotective role of phytochemicals. Molecules. 2018; 23:2485. PMID: 30262792.26. Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res. 2015; 29:323–331. PMID: 25394264.27. Morin B, Nichols LA, Zalasky KM, Davis JW, Manthey JA, Holland LJ. The citrus flavonoids hesperetin and nobiletin differentially regulate low density lipoprotein receptor gene transcription in HepG2 liver cells. J Nutr. 2008; 138:1274–1281. PMID: 18567747.28. Marletta MA. Nitric oxide synthase structure and mechanism. J Biol Chem. 1993; 268:12231–12234. PMID: 7685338.29. Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011; 21:103–115. PMID: 21187859.30. Yue J, López JM. Understanding MAPK signaling pathways in apoptosis. Int J Mol Sci. 2020; 21:2346. PMID: 32231094.31. Larson EB. Prospects for delaying the rising tide of worldwide, late-life dementias. Int Psychogeriatr. 2010; 22:1196–1202. PMID: 20594386.32. Teleanu DM, Niculescu AG, Lungu II, Radu CI, Vladâcenco O, Roza E, Costăchescu B, Grumezescu AM, Teleanu RI. An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int J Mol Sci. 2022; 23:5938. PMID: 35682615.33. Nita M, Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev. 2016; 2016:3164734. PMID: 26881021.34. Liu JT, Dong MH, Zhang JQ, Bai Y, Kuang F, Chen LW. Microglia and astroglia: the role of neuroinflammation in lead toxicity and neuronal injury in the brain. Neuroimmunol Neuroinflamm. 2015; 2:131–137.35. Amato A, Terzo S, Mulè F. Natural compounds as beneficial antioxidant agents in neurodegenerative disorders: a focus on Alzheimer’s disease. Antioxidants. 2019; 8:608. PMID: 31801234.36. Shimmyo Y, Kihara T, Akaike A, Niidome T, Sugimoto H. Epigallocatechin-3-gallate and curcumin suppress amyloid beta-induced beta-site APP cleaving enzyme-1 upregulation. Neuroreport. 2008; 19:1329–1333. PMID: 18695518.37. Song X, Liu B, Cui L, Zhou B, Liu W, Xu F, Hayashi T, Hattori S, Ushiki-Kaku Y, Tashiro SI, et al. Silibinin ameliorates anxiety/depression-like behaviors in amyloid β-treated rats by upregulating BDNF/TrkB pathway and attenuating autophagy in hippocampus. Physiol Behav. 2017; 179:487–493. PMID: 28735062.38. Kumar V, Giacobini E. Cerebrospinal fluid choline, and acetylcholinesterase activity in familial vs. non-familial Alzheimer’s disease patients. Arch Gerontol Geriatr. 1988; 7:111–117. PMID: 3415394.39. Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015; 124:64–74. PMID: 25625242.40. Elstner EF. Oxygen activation and oxygen toxicity. Annu Rev Plant Physiol. 1982; 33:73–96.41. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000; 408:239–247. PMID: 11089981.42. Ma J, Gao SS, Yang HJ, Wang M, Cheng BF, Feng ZW, Wang L. Neuroprotective effects of proanthocyanidins, natural flavonoids derived from plants, on rotenone-induced oxidative stress and apoptotic cell death in human neuroblastoma SH-SY5Y cells. Front Neurosci. 2018; 12:369. PMID: 29904339.43. Ling APK, Chan HH, Koh RY, Wong YP. Neuroprotective roles of asiaticoside on hydrogen peroxide-induced toxicity in SH-SY5Y cells. J Fundam Appl Sci. 2017; 9:636–649.44. Andreasson KI, Bachstetter AD, Colonna M, Ginhoux F, Holmes C, Lamb B, Landreth G, Lee DC, Low D, Lynch MA, et al. Targeting innate immunity for neurodegenerative disorders of the central nervous system. J Neurochem. 2016; 138:653–693. PMID: 27248001.45. Mukaida N, Ishikawa Y, Ikeda N, Fujioka N, Watanabe S, Kuno K, Matsushima K. Novel insight into molecular mechanism of endotoxin shock: biochemical analysis of LPS receptor signaling in a cell-free system targeting NF-κB and regulation of cytokine production/action through β2 integrin in vivo . J Leukoc Biol. 1996; 59:145–151. PMID: 8603986.46. Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017; 2:17023. PMID: 29158945.47. Schmedtje JF Jr, Ji YS, Liu WL, DuBois RN, Runge MS. Hypoxia induces cyclooxygenase-2 via the NF-κB p65 transcription factor in human vascular endothelial cells. J Biol Chem. 1997; 272:601–608. PMID: 8995303.48. Baldwin AS Jr. The NF-κB and I κB proteins: new discoveries and insights. Annu Rev Immunol. 1996; 14:649–683. PMID: 8717528.49. Park JH, Kim SH, Lee SR. Inhibitory effect of Petalonia binghamiae on neuroinflammation in LPS-stimulated microglial cells. J Nutr Health. 2017; 50:25–31.50. Shal B, Ding W, Ali H, Kim YS, Khan S. Anti-neuroinflammatory potential of natural products in attenuation of Alzheimer’s disease. Front Pharmacol. 2018; 9:548. PMID: 29896105.51. Naoi M, Shamoto-Nagai M, Maruyama W. Neuroprotection of multifunctional phytochemicals as novel therapeutic strategy for neurodegenerative disorders: antiapoptotic and antiamyloidogenic activities by modulation of cellular signal pathways. Future Neurol. 2019; 14:FNL9.52. Park CH, Kim JH, Choi SH, Shin YS, Lee SW, Cho EJ. Protective effects of Glycyrrhiza uralensis Radix extract and Its active compounds on H2O2-induced apoptosis of C6 glial cells. Korean J Med Crop Sci. 2017; 25:315–321.53. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007; 35:495–516. PMID: 17562483.54. Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996; 8:402–411. PMID: 8793994.55. Ruffels J, Griffin M, Dickenson JM. Activation of ERK1/2, JNK and PKB by hydrogen peroxide in human SH-SY5Y neuroblastoma cells: role of ERK1/2 in H2O2-induced cell death. Eur J Pharmacol. 2004; 483:163–173. PMID: 14729104.56. Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J Signal Transduct. 2011; 2011:792639. PMID: 21637379.57. Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J. 2010; 277:2–21. PMID: 19843174.58. Subramaniam S, Unsicker K. ERK and cell death: ERK1/2 in neuronal death. FEBS J. 2010; 277:22–29. PMID: 19843173.59. Ouyang M, Shen X. Critical role of ASK1 in the 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J Neurochem. 2006; 97:234–244. PMID: 16515547.60. Kim SD, Moon CK, Eun SY, Ryu PD, Jo SA. Identification of ASK1, MKK4, JNK, c-Jun, and caspase-3 as a signaling cascade involved in cadmium-induced neuronal cell apoptosis. Biochem Biophys Res Commun. 2005; 328:326–334. PMID: 15670787.61. Feng HJ, Bao YL, Liang ZP, Zhao FP, Xu SE, Xu W, Zhao C, Qin G. Silencing of FANCD2 enhances the radiosensitivity of metastatic cervical lymph node-derived head and neck squamous cell carcinoma HSC-4 cells. Int J Oncol. 2017; 50:1241–1250. PMID: 28350060.62. Su JC, Lin KL, Chien CM, Lu CM, Chen YL, Chang LS, Lin SR. Novel indoloquinoline derivative, IQDMA, induces G2/M phase arrest and apoptosis in A549 cells through JNK/p38 MAPK signaling activation. Life Sci. 2009; 85:505–516. PMID: 19699753.63. Lee S, Youn K, Jeong WS, Ho CT, Jun M. Protective effects of red ginseng oil against Aβ25-35-induced neuronal apoptosis and inflammation in PC12 cells. Int J Mol Sci. 2017; 18:2218. PMID: 29065557.64. Tang S, Li C, Suo Z, Xu Y, Wei K, Zhao L, Huang H, Liu X, Liu D, Li X. Neuroprotective effects of savinin on LPS-induced neuroinflammation in vivo via regulating MAPK/NF-κB pathway and NLRP3 inflammasome activation. Molecules. 2023; 28:1575. PMID: 36838564.65. Wang MM, Feng YS, Yang SD, Xing Y, Zhang J, Dong F, Zhang F. The relationship between autophagy and brain plasticity in neurological diseases. Front Cell Neurosci. 2019; 13:228. PMID: 31244604.66. Kim HJ, Kim J, Kang KS, Lee KT, Yang HO. Neuroprotective effect of chebulagic acid via autophagy induction in SH-SY5Y cells. Biomol Ther (Seoul). 2014; 22:275–281. PMID: 25143804.67. Shirgadwar SM, Kumar R, Preeti K, Khatri DK, Singh SB. Neuroprotective effect of phloretin in rotenone-induced mice model of Parkinson’s disease: modulating mTOR-NRF2-p62 mediated autophagy-oxidative stress crosstalk. J Alzheimers Dis. Forthcoming. 2022.68. Xi X, Zou C, Ye Z, Huang Y, Chen T, Hu H. Pioglitazone protects tubular cells against hypoxia/reoxygenation injury through enhancing autophagy via AMPK-mTOR signaling pathway. Eur J Pharmacol. 2019; 863:172695. PMID: 31560869.69. Hill JL, Kobori N, Zhao J, Rozas NS, Hylin MJ, Moore AN, Dash PK. Traumatic brain injury decreases AMP-activated protein kinase activity and pharmacological enhancement of its activity improves cognitive outcome. J Neurochem. 2016; 139:106–119. PMID: 27379837.70. Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002; 99:467–472. PMID: 11756682.71. Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010; 33:67–75. PMID: 19963289.72. Ravikumar B, Berger Z, Vacher C, O’Kane CJ, Rubinsztein DC. Rapamycin pre-treatment protects against apoptosis. Hum Mol Genet. 2006; 15:1209–1216. PMID: 16497721.73. Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012; 13:251–262. PMID: 22436748.74. Wang Z, Zhou L, Zheng X, Chen G, Pan R, Li J, Liu W. Autophagy protects against PI3K/Akt/mTOR-mediated apoptosis of spinal cord neurons after mechanical injury. Neurosci Lett. 2017; 656:158–164. PMID: 28739349.75. Stacchiotti A, Corsetti G. Natural compounds and autophagy: allies against neurodegeneration. Front Cell Dev Biol. 2020; 8:555409. PMID: 33072744.76. Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals. 2011; 19:163–174. PMID: 21778691.77. Chen J, Deng X, Liu N, Li M, Liu B, Fu Q, Qu R, Ma S. Quercetin attenuates tau hyperphosphorylation and improves cognitive disorder via suppression of ER stress in a manner dependent on AMPK pathway. J Funct Foods. 2016; 22:463–476.78. Zhao Y, Wang Q, Wang Y, Li J, Lu G, Liu Z. Glutamine protects against oxidative stress injury through inhibiting the activation of PI3K/Akt signaling pathway in parkinsonian cell model. Environ Health Prev Med. 2019; 24:4. PMID: 30611190.79. Wu Y, Cui J. (-)-Epigallocatechin-3-gallate provides neuroprotection via AMPK activation against traumatic brain injury in a mouse model. Naunyn Schmiedebergs Arch Pharmacol. 2020; 393:2209–2220. PMID: 32062732.80. Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies P, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-β peptide metabolism. J Biol Chem. 2010; 285:9100–9113. PMID: 20080969.81. Tramutola A, Triplett JC, Di Domenico F, Niedowicz DM, Murphy MP, Coccia R, Perluigi M, Butterfield DA. Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J Neurochem. 2015; 133:739–749. PMID: 25645581.82. Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, O’Connor R, O’Neill C. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem. 2005; 93:105–117. PMID: 15773910.83. Wang Y, Ma Q, Ma X, Zhang Z, Liu N, Wang M. Role of mammalian target of rapamycin signaling in autophagy and the neurodegenerative process using a senescence accelerated mouse-prone 8 model. Exp Ther Med. 2017; 14:1051–1057. PMID: 28810557.84. Gao L, Li X, Meng S, Ma T, Wan L, Xu S. Chlorogenic acid alleviates Aβ25-35-induced autophagy and cognitive impairment via the mTOR/TFEB signaling pathway. Drug Des Devel Ther. 2020; 14:1705–1716.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroprotective effects of resveratrol on 6-hydroxydopamine-induced damage of SH-SY5Y cell line
- Protective effects of mealworm (Tenebrio molitor) extract on N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)–induced cellular toxicity in SH-SY5Y neuroblastoma cells
- Neuroprotective effect of Deodeok (Codonopsis lanceolata) bud extracts in H2O2 -stimulated SH-SY5Y cells
- Neuregulin-1 Protects Neuronal Cells Against Damage due to CoCl2-Induced Hypoxia by Suppressing Hypoxia-Inducible Factor-1α and P53 in SH-SY5Y Cells
- Neuroprotective effect of fermented ginger extracts by Bacillus subtilis in SH-SY5Y cells