J Korean Med Sci.
2023 Oct;38(40):e313. 10.3346/jkms.2023.38.e313.
The Association Between Tachycardia and Mortality in Septic Shock Patients According to Serum Lactate Level: A Nationwide Multicenter Cohort Study
- Affiliations
-
- 1Department of Critical Care Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Pulmonary, Allergy and Critical Care Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea
- 4Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 5Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2546931
- DOI: http://doi.org/10.3346/jkms.2023.38.e313
Abstract
- Background
This study aimed to evaluate whether the effect of tachycardia varies according to the degree of tissue perfusion in septic shock.
Methods
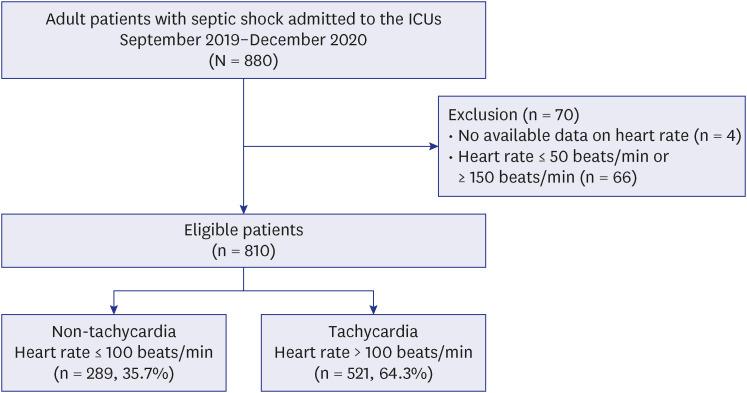
Patients with septic shock admitted to the intensive care units were categorized into the tachycardia (heart rate > 100 beats/min) and non-tachycardia (≤ 100 beats/min) groups. The association of tachycardia with hospital mortality was evaluated in each subgroup with low and high lactate levels, which were identified through a subpopulation treatment effect pattern plot analysis.
Results
In overall patients, hospital mortality did not differ between the two groups (44.6% vs. 41.8%, P = 0.441), however, tachycardia was associated with reduced hospital mortality rates in patients with a lactate level ≥ 5.3 mmol/L (48.7% vs. 60.3%, P = 0.030; adjusted odds ratio [OR], 0.59, 95% confidence interval [CI], 0.35–0.99, P = 0.045), not in patients with a lactate level < 5.3 mmol/L (36.5% vs. 29.7%, P = 0.156; adjusted OR, 1.39, 95% CI, 0.82–2.35, P = 0.227).
Conclusion
In septic shock patients, the effect of tachycardia on hospital mortality differed by serum lactate level. Tachycardia was associated with better survival in patients with significantly elevated lactate levels.
Keyword
Figure
Reference
-
1. Carrara M, Ferrario M, Bollen Pinto B, Herpain A. The autonomic nervous system in septic shock and its role as a future therapeutic target: a narrative review. Ann Intensive Care. 2021; 11(1):80. PMID: 33999297.2. Heusch G. Heart rate in the pathophysiology of coronary blood flow and myocardial ischaemia: benefit from selective bradycardic agents. Br J Pharmacol. 2008; 153(8):1589–1601. PMID: 18223669.3. Sander O, Welters ID, Foëx P, Sear JW. Impact of prolonged elevated heart rate on incidence of major cardiac events in critically ill patients with a high risk of cardiac complications. Crit Care Med. 2005; 33(1):81–88. PMID: 15644652.4. Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013; 310(16):1683–1691. PMID: 24108526.5. Hasegawa D, Sato R, Prasitlumkum N, Nishida K, Takahashi K, Yatabe T, et al. Effect of ultrashort-acting β-blockers on mortality in patients with sepsis with persistent tachycardia despite initial resuscitation: a systematic review and meta-analysis of randomized controlled trials. Chest. 2021; 159(6):2289–2300. PMID: 33434497.6. Kakihana Y, Nishida O, Taniguchi T, Okajima M, Morimatsu H, Ogura H, et al. Efficacy and safety of landiolol, an ultra-short-acting β1-selective antagonist, for treatment of sepsis-related tachyarrhythmia (J-Land 3S): a multicentre, open-label, randomised controlled trial. Lancet Respir Med. 2020; 8(9):863–872. PMID: 32243865.7. Russell A, Rivers EP, Giri PC, Jaehne AK, Nguyen HB. A physiologic approach to hemodynamic monitoring and optimizing oxygen delivery in shock resuscitation. J Clin Med. 2020; 9(7):2052. PMID: 32629778.8. Na SJ, Oh DK, Park S, Lee YJ, Hong SB, Park MH, et al. Clinical characteristics and outcomes of neutropenic sepsis: a multicenter cohort study. Shock. 2022; 57(5):659–665. PMID: 35066514.9. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016; 315(8):801–810. PMID: 26903338.10. Pramanik D. Cardiac output. Principles of Physiology. 5th ed. New Delhi, India: Jaypee Brothers Medical Publishers;2015. p. 263–271.11. Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med. 2018; 44(6):925–928. PMID: 29675566.12. Bonetti M, Gelber RD. Patterns of treatment effects in subsets of patients in clinical trials. Biostatistics. 2004; 5(3):465–481. PMID: 15208206.13. Liu LC, Valente MA, Postmus D, O’Connor CM, Metra M, Dittrich HC, et al. Identifying subpopulations with distinct response to treatment using plasma biomarkers in acute heart failure: results from the PROTECT trial: differential response in acute heart failure. Cardiovasc Drugs Ther. 2017; 31(3):281–293. PMID: 28656542.14. D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998; 17(19):2265–2281. PMID: 9802183.15. Rosenbaum PR. Optimal matching for observational studies. J Am Stat Assoc. 1989; 84(408):1024–1032.16. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014; 12(12):1495–1499. PMID: 25046131.17. Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993; 328(20):1471–1477. PMID: 8479467.18. Ostrowski SR, Gaïni S, Pedersen C, Johansson PI. Sympathoadrenal activation and endothelial damage in patients with varying degrees of acute infectious disease: an observational study. J Crit Care. 2015; 30(1):90–96. PMID: 25457118.19. Pancoto JA, Corrêa PB, Oliveira-Pelegrin GR, Rocha MJ. Autonomic dysfunction in experimental sepsis induced by cecal ligation and puncture. Auton Neurosci. 2008; 138(1-2):57–63. PMID: 18060845.20. Andreis DT, Singer M. Catecholamines for inflammatory shock: a Jekyll-and-Hyde conundrum. Intensive Care Med. 2016; 42(9):1387–1397. PMID: 26873833.21. Hoke RS, Müller-Werdan U, Lautenschläger C, Werdan K, Ebelt H. Heart rate as an independent risk factor in patients with multiple organ dysfunction: a prospective, observational study. Clin Res Cardiol. 2012; 101(2):139–147. PMID: 22048696.22. Wilson J, Higgins D, Hutting H, Serkova N, Baird C, Khailova L, et al. Early propranolol treatment induces lung heme-oxygenase-1, attenuates metabolic dysfunction, and improves survival following experimental sepsis. Crit Care. 2013; 17(5):R195. PMID: 24020447.23. Morelli A, Donati A, Ertmer C, Rehberg S, Kampmeier T, Orecchioni A, et al. Microvascular effects of heart rate control with esmolol in patients with septic shock: a pilot study. Crit Care Med. 2013; 41(9):2162–2168. PMID: 23873274.24. Morelli A, Singer M, Ranieri VM, D’Egidio A, Mascia L, Orecchioni A, et al. Heart rate reduction with esmolol is associated with improved arterial elastance in patients with septic shock: a prospective observational study. Intensive Care Med. 2016; 42(10):1528–1534. PMID: 27101380.25. Levy B, Fritz C, Piona C, Duarte K, Morelli A, Guerci P, et al. Hemodynamic and anti-inflammatory effects of early esmolol use in hyperkinetic septic shock: a pilot study. Crit Care. 2021; 25(1):21. PMID: 33413583.26. Zanotti Cavazzoni SL, Dellinger RP. Hemodynamic optimization of sepsis-induced tissue hypoperfusion. Crit Care. 2006; 10(Suppl 3):Suppl 3. S2.27. Yamashita S, Suzuki T, Iguchi K, Sakamoto T, Tomita K, Yokoo H, et al. Cardioprotective and functional effects of levosimendan and milrinone in mice with cecal ligation and puncture-induced sepsis. Naunyn Schmiedebergs Arch Pharmacol. 2018; 391(9):1021–1032. PMID: 29922941.28. Desai R, Hanna B, Singh S, Omar A, Deshmukh A, Kumar G, et al. Trends and outcomes in sepsis hospitalizations with and without atrial fibrillation: a nationwide inpatient analysis. Crit Care Med. 2019; 47(8):e630–e638. PMID: 31094740.29. Landesberg G, Gilon D, Meroz Y, Georgieva M, Levin PD, Goodman S, et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur Heart J. 2012; 33(7):895–903. PMID: 21911341.30. Lanspa MJ, Cirulis MM, Wiley BM, Olsen TD, Wilson EL, Beesley SJ, et al. Right ventricular dysfunction in early sepsis and septic shock. Chest. 2021; 159(3):1055–1063. PMID: 33068615.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Serum lactate levels in cirrhosis and non-cirrhosis patients with septic shock
- Lactate Clearance and Outcome in Septic Shock Patients with Low Level of Initial Lactate
- Utility of the early lactate area score as a prognostic marker for septic shock patients in the emergency department
- Recent lactate findings: is repeated serum lactate testing necessary in septic shock patients?
- Lactate Clearance as a Prognostic Factor of Organ Failure in Sepsis